Abstract: Innate immunity is activated when the pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs). We show that a liver-derived secretory protein LECT2, a hepatokine, binds to the HGF receptor MET, a proto-oncogene product, to suppress the proliferation signal of MET. LECT2 activates retinoic acid-inducible gene-I, enhancing antiviral and innate immune responses through MET. Thus, LECT2 is an anti-proliferative and immunoregulatory factor that could be a therapeutic target for viral infections and cancer.
[Background]
The liver is the largest organ in the human body. It is suggested that hepatokines secreted from the liver play important roles in hepatocytes as well as immunocompetent cells in other organs. In this study, the research team of Kanazawa University focused on LECT2*1), a hepatokine, and conducted research aimed at elucidating the mechanism of action of LECT2 on hepatocytes for the purpose of exploring the possibility of new treatment methods.
RIG-I*2) is an important innate immune sensor that recognizes viral RNA and promotes interferon (IFN) production. In recent years, it has been reported that host RNA binds to RIG-I and regulates its activation. The research team previously showed that the mRNA of selenoprotein P, one of the hepatokines, binds to RIG-I and negatively regulates the innate immune responses (Murai, Honda, et al., Cell Host & Microbe, 2019). However, how host proteins regulate RIG-I activation is not sufficiently understood. Therefore, in this study, the team focused on LECT2 and investigated how LECT2 contributes to the innate immune responses upon viral infection.
[Results]
First, the team found that the expression of type I IFN and IFN-stimulated genes was significantly enhanced in cells treated with the LECT2 protein upon viral infection. As a result, the virus replication was significantly suppressed in cells treated with the LECT2 protein. Interestingly, induction of RIG-I expression was observed in cells treated with LECT2, while induction of the expression of other PRRs*3) such as MDA5 was not observed. Next, the team created LECT2 transgenic mice (LECT2-TG) and knockout mice (LECT2-KO), and infected these mice with lymphocytic choriomeninitis virus (LCMV). It was found that LCMV replication was markedly decreased in the LECT2-TG mice while it was markedly increased in the LECT2-KO mice. These results clearly indicate that LECT2 is an important hepatokine for virus elimination.
LECT2 is known to bind to the HGF receptor MET, the product of a proto-oncogene. Therefore, the team examined how MET itself would affect the innate immune responses. Interestingly, the viral RNA-induced expression of type I IFN was markedly suppressed in cells in which MET had been knocked out. Furthermore, LCMV replication was significantly increased in mice in which MET gene expression was suppressed by antisense oligo (Gapmer) compared to mice in which control Gapmer had been introduced. These results clarified that MET itself played important roles for the innate immune responses.
In addition, the team examined which phosphorylation site of MET was affected by the binding of LECT2 to MET and revealed that LECT2 specifically suppresses the 1349th tyrosine phosphorylation of MET. It is known that adapters such as Gab1 and SHP2 bind to the 1349th tyrosine phosphorylation site of MET and are involved in downstream signaling. LECT2 was found to negatively regulate SHP2 activity by inhibiting the 1349th tyrosine phosphorylation of MET. The SHP2/c-Cbl complex is involved in the proteasome degradation of RIG-I. LECT2 inhibited the degradation of RIG-I by SHP2/c-Cbl by inactivating SHP2. Moreover, the team revealed that the binding of phosphatase PTP4A1*4) specifically to the 1349th tyrosine phosphorylation site was essential for LECT2 to induce dephosphorylation of the 1349th tyrosine of MET.
[Future prospects]
New coronavirus infections (COVID-19) have spread seriously around the globe and have claimed many lives. Elucidation of the viral infection mechanism and that of the immune responses upon viral infection are essential for the development of new antiviral therapies and preventive methods. In this study, the team was able to propose a new, previously unrecognized concept that MET-mediated proliferation and innate immunity were closely interacted via LECT2-MET. The results of the present study are expected to contribute to the construction of new therapeutic strategies for infectious diseases and cancer targeting LECT2.
[Glossary]
*1) LECT2 (leukocyte cell-derived chemotaxin 2)
LECT2 was identified as a protein with neutrophil chemotactic activity. Nowadays, LECT2 is suggested to be a multifunctional protein.
*2) RIG-I (retinoic acid-inducible gene-I)
RIG-I is a protein molecule that works in the innate immune system. When a virus enters the cell, RIG-I recognizes virus-derived RNA and induces the production of type I interferon, which has antiviral activity.
*3) PRR (pattern recognition receptor)
PRRs are receptors that recognize common molecular patterns of viruses and microorganisms such as bacteria, including Toll-like receptors, NOD-like receptors, RIG-I-like receptors, and C-type lectin receptors.
*4) PTP4A1 (protein tyrosine phosphatase 4A1)
PTP4A1 belongs to the class of prenylated protein tyrosine phosphatases (PTPs) and is a protein containing the PTP domain and the characteristic C-terminal prenylation motif.
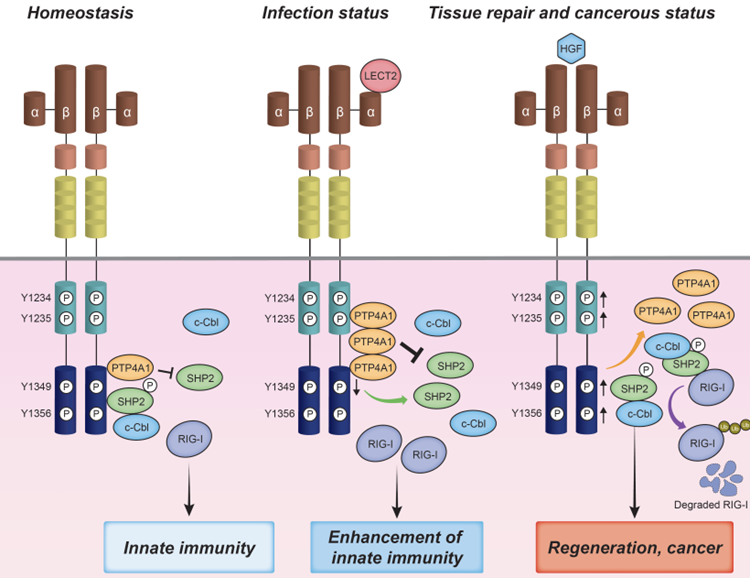
Conceptual diagram of the present study
LECT2 suppresses the recruitment of SHP2/c-Cbl to MET, which is known to promote proteasome degradation of RIG-I, by selectively suppressing the phosphorylation of the 1349th tyrosine of the HGF receptor MET. LECT2 promotes the recruitment of the second protein tyrosine phosphatase, PTP4A1, to MET and PTP4A1 dephosphorylates SHP2 under ligand-free conditions. Thus, PTP4A1 and SHP2 have antagonistic effects on MET, and the antagonism of these phosphatases that bind to MET is essential for maintaining a normal homeostatic balance. When cells are infected with a virus, LECT2 functions as the primary ligand for MET, shifting the balance to the involvement of PTP4A1 and resulting in enhanced RIG-I-dependent innate immune responses. On the other hand, in tissue repair and cancer, HGF is the main ligand for MET, suppressing the innate immune responses and enhancing the growth signal. The research team has revealed the existence of crosstalk between the innate immune responses and MET-dependent proliferation in the liver.
[Funder]
Japan Agency for Medical Research and Development (AMED)
[Grant numbers]
JP20fk0210046, JP22fk0210073, JP22fk0310514, JP22fk0210112, JP21fk0210048, JP22fk0210095
[Article]
Title: Leukocyte cell-derived chemotaxin 2 is an antiviral regulator acting through the proto-oncogene MET
Journal: Nature Communications
Authors: Takayoshi SHIRASAKI, Satoshi YAMAGOE, Tetsuro SHIMAKAMI, Kazuhisa MURAI, Ryu IMAMURA, Kiyo-Aki ISHII, Hiroaki TAKAYAMA, Yukako MATSUMOTO, Natsumi TAJIMA-SHIRASAKI, Naoto NAGATA, Ryogo SHIMIZU, Souma YAMANAKA, Atsushi ABE, Hitoshi OMURA, Kazunori KAWAGUCHI, Hikari OKADA, Taro YAMASHITA, Tomoki YOSHIKAWA, Kazuhiro TAKIMOTO, Motoko TAHARAGUCHI, Shogo TAKATSUKA, Yoshitsugu MIYAZAKI, Toshikatsu TAMAI, Yamato TANABE, Makoto KURACHI, Yasuhiko YAMAMOTO, Shuichi KANEKO, Kunio MATSUMOTO, Toshinari TAKAMURA, Masao HONDA
DOI: 10.1038/s41467-022-30879-3
Published online on June 8, 2022



 PAGE TOP
PAGE TOP