Abstract:
Hepatocyte Growth Factor (HGF) regulates tissue regeneration and cancer progression, and is a drug candidate for treatment of ALS (amyotrophic lateral sclerosis). A panel of functionally different monoclonal antibodies to HGF has successfully been generated. Among them, some antibodies specifically recognize mature HGF and inhibit HGF activity. Use of these monoclonal antibodies has unique significance to elucidate molecular structures, activation, and signaling mechanism of HGF.
Hepatocyte growth factor (HGF) was discovered by Japanese scientists in mid 80s, and is known to stimulate biological activities such as cell proliferation, survival and migration upon binding to its receptor, Met. Since HGF also stimulates survival of motor neurons, clinical trial of recombinant HGF for treatment of ALS (amyotrophic lateral sclerosis) has been performed at Tohoku University and Osaka University. On the other hand, since aberrant activation of Met receptor is related with cancer progression, research on Met-inhibitors and on antibody drugs that would inhibit HGF binding to Met receptor is now actively being carried out.
HGF is secreted as a precursor protein of single-chain polypeptide without activity, but is cleaved by a certain protease to become two-chain polypeptide protein, the mature form with HGF activity. The precursor protein of single-chain polypeptide as well as the mature form with two-chain polypeptides bind to Met receptor. However, only the two-chain HGF binds and activates the Met receptor. It has been unknown what kind of structural changes would take place during the processing/cleavage from the single-chain precursor HGF to two-chain active HGF.
The collaborative research team from Osaka University, Tohoku University, and Kanazawa University is challenging to this important problem. In their approach, noncleavable single-chain HGF (devoid of two-chain HGF) and two-chain HGF (devoid of single-chain HGF) were separately prepared by recombinant protein expression and purification. By using these single-chain/two-chain HGF, 6 different monoclonal antibodies were raised; for example, among them t8E4 the antibody recognizes the structural difference between the HGF precursor and the mature form, and t1E4 antibody strongly inhibits Met activation.
These functionally different monoclonal antibodies enable the elucidation of crystal structures and structural bases for activation and signaling driven by of HGF-Met pathway. It is also expected that the current study will contribute to development of novel antibody drugs for ALS and cancer.
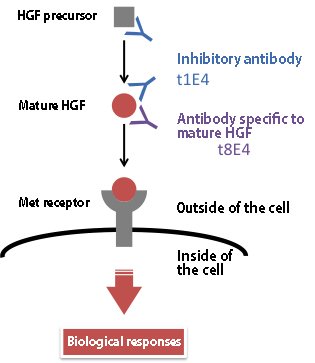
Figure 1. Antibodies to HGF raised in this study; the antibody inhibitory to HGF activities and the antibody specific to mature HGF.
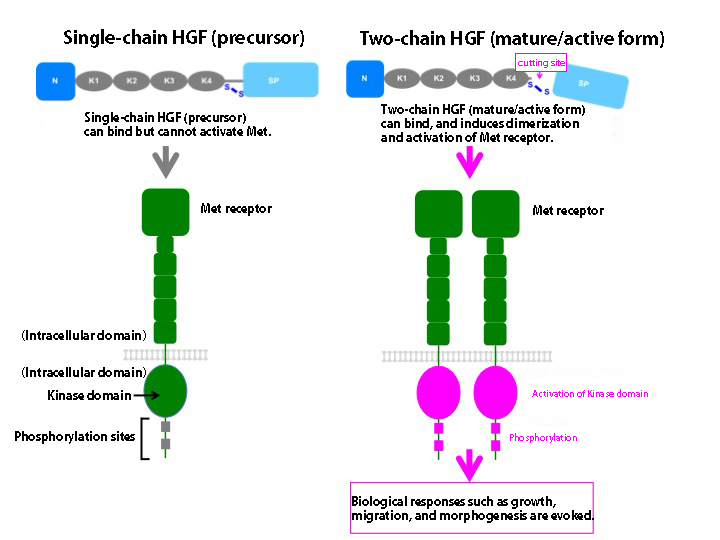
Figure 2. Mechanism of HGF activation
Article
Title: Probing conformational and functional states of human hepatocyte growth factor by a panel of monoclonal antibodies
Journal: Scientific Reports 6
Authors: Masataka UMITSU1, Katsuya SAKAI2, Satoshi OGASAWARA3, Mika K. KANEKO3, Ryoko ASAKI1, Keiko TAMURA-KAWAKAMI1, Yukinari KATO3, Kunio MATSUMOTO2, Junichi TAKAGI1
1Institute for Protein Research, Osaka University, 2Cancer Research Institute, Kanazawa University, 3Graduate School of Medicine, Tohoku University
Doi: 10.1038/srep33149
Funder
Grant-in-Aid for Scientific Research on Innovative Areas from MEXT to J.T.; “Platform for Drug Discovery, Informatics, and Structural Life Science” grant from AMED to J.T. and Y.K.; “X-ray Free Electron Laser Priority Strategy Program” from MEXT to J.T.; Regional Innovation Strategy Support Program from MEXT to Y.K.; Basic Science and Platform Technology Program for Innovative Biological Medicine from AMED to Y.K.; Grants-in-Aid from JSPS to K.M. and K.S.



 PAGE TOP
PAGE TOP