Abstract:
A scanning electrochemical cell imaging technique shows how nanoscale structural features affect the catalytic activity of MoS2 monolayers for hydrogen evolution reactions, report researchers at Kanazawa University in Angewandte Chemie International Edition.
The properties of 2D transition metal dichalcogenides are attracting a great deal of interest, and one of the reasons is their catalytic activity. In particular, better catalysts are needed to exploit the potential of water electrolysis – splitting water into its component elements – to provide sustainable energy storage.
"MoS2 is one of the most promising precious rare metal-free catalysts for the hydrogen evolution reaction (HER)," point out Yasufumi Takahashi, Mingwei Chen, and Tomokazu Matsue and their colleagues at Kanazawa University and other collaborating institutions in Japan, the US and the UK in their recent Angewandte Chemie International Edition report. The work highlights the role of “scanning electrochemical cell microscopy” for engineering the catalytic properties of these 2D materials.
As the researchers point out, scanning electrochemical microscopy has already proved useful in investigations of the catalytic activity of MoS2 monolayers, which have focused on the effects of strain, as well as the metallic versus semiconducting properties of different microstructural phases of MoS2 on HER catalysis. These studies used a microscale electrode to probe the sample for electrochemical activity as a function of location with high spatial resolution, on account of the microscale dimensions of the electrode.
In their scanning electrochemical cell microscopy studies, Takahashi, Chen, Matsue and colleagues use a nanopipette as a local, moveable electrochemical cell to probe the electrochemical activity on the surface instead of an ultramicroelectrode. They highlight the “reproducible and reliable technique for fabricating nanoprobes together with fast electrochemical characterization due to its small capacitive current” as additional advantages of this form of the characterization technique.
The researchers used a nanopipette with a 20 nm radius to study triangular monolayers of MoS2 with a 1H microstructural phase, as well as heterostructures of MoS2 and WS2. Each flake had a side length of around 130 nm. The measurements revealed changes in catalytic activity where edges, terrace features and heterojunctions between MoS2 and WS2 were located, which agrees with the suggestions of previous reports. In addition, aging the sample had a noticeable effect, particularly at edges.
The researchers conclude that their study demonstrates how it is possible to evaluate the local HER activity of catalytic samples using scanning electrochemical cell microscopy. They suggest that the technique can be a “powerful tool” for engineering the phase and structure of 2D transition metal dichalcogenide samples for applications in catalysis.
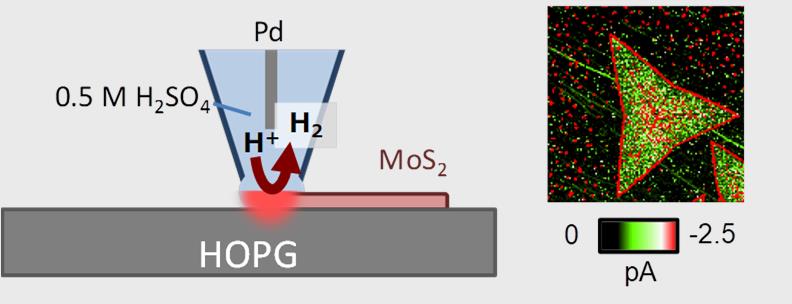
Figure 1. [TOC image]
Scanning electrochemical cell microscopy (SECCM) allows imaging and quantitative analysis of hydrogen evolution reaction (HER) catalytically active sites in 1H MoS2 monolayers.
[Background]
2D transition metal dichalcogenides
The isolation of graphene and the extraordinary properties identified in the material attracted intense interest from researchers not only in graphene but in a whole host of other materials, where 2D layers could be isolated. Among these 2D materials are transition metal dichalcogenides where the transition metals include molybdenum (Mo) and tungsten (W) and the chalcogens are group VI elements, which include sulfur (S), selenium (Se) and tellurium (Te). As well as electrochemical catalysis for energy storage, this group of materials has also attracted interest for high-end electronics, spintronics, optoelectronics, energy harvesting, flexible electronics, DNA sequencing and personalized medicine.
The hydrogen evolution reaction
The use of hydrogen as a fuel involves burning it in oxygen to produce just water and the release of a lot of energy. Hydrogen fuel avoids the use of fossil fuels and the production of carbon dioxide, and it can get around some of the issues of energy storage associated with many alternative sustainable energy technologies such as solar and wind power. The electrolysis of water using a sustainably sourced current provides an environmentally friendly way of producing hydrogen fuel. Although HER is faster than the oxygen evolution reaction, there is still great interest in increasing the reaction rates. As a result, there is a lot of interest in the catalytic activity of 2D transition metal dichalcogenides on HER among other reactions.
Article
High Resolution Electrochemical Mapping of Hydrogen Evolution Reaction on Transition Metal Dichalcogenide Nanosheets
Journal: Angewandte Chemie International Edition
Authors: Yasufumi Takahashi, Yu Kobayashi, Ziqian Wang, Yoshikazu Ito, Masato Ota, Hiroki Ida, Akichika Kumatani, Keisuke Miyazawa, Takeshi Fujita, Hitoshi Shiku, Yuri E. Korchev, Yasumitsu Miyata, Takeshi Fukuma, Mingwei Chen and Tomokazu Matsue
Funder
World Premier International Research Center Initiative (WPI), MEXT, Japan; Development of Systems and Technology for Advanced Measurement and Analysis from AMED (The Japan Agency for Medical Research and Development)-SENTAN; ALCA, PRESTO (JPMJPR14FA, JPMJPR18T8, JPMJPR1541), CREST (JPMJCR16F3, JPMJCR11C5) from the Japan Science and Technology Agency (JST); a Grant-in-Aid for Scientific Research (A) (16H02280, 19H00915), a Grant-in-Aid for Scientific Research (B) (15H03542, 18H01832), a Grant-in-Aid for Young Scientists (A) (15H05422 and 16H06042), a Grant-in-Aid for Exploratory Research (15K13263 and 17K19135), and a Grant-in-Aid for Scientific Research on Innovative Areas (16H00885) from the Japan Society for the Promotion of Science (JSPS); Asahi Glass Foundation; Grant-in-Aid for Young Scientists provided by Hokuriku bank; Murata Science Foundation; Intelligent Cosmos Academic Foundation are all thankfully acknowledged for financial support. This work is partially sponsored by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research on Innovative Areas "Science of Atomic Layers" (Grant JP26107504).



 PAGE TOP
PAGE TOP