Abstract:
Sodium-glucose cotransporter 2 inhibitor (SGLT2i) reduces blood glucose levels in type 2 diabetes but increases hepatic gluconeogenesis, offsetting its glucose-lowering effect. To elucidate the SGLT2i effect and its mechanism, we generated a mouse model showing liver-specific expression of Gaussia luciferase driven by the gluconeogenic enzyme gene G6pc promoter. Single SGLT2i administration increased the hepatic gluconeogenic response in lean insulin-sensitive mice, but not in obese insulin-resistant mice. Long-term SGLT2i administration relieved obesity-induced upregulation of the hepatic gluconeogenic response by restoring impeded hepatic insulin signaling in obese insulin-resistant mice.
[Background]
Diabetes is a metabolic disorder characterized by a high blood glucose level exceeding normal values over a prolonged period. Type-2 diabetes, accounting for about 90% of diabetes patients, is due to lifestyle and genetic factors but could be prevented. However, once it develops, it is difficult to cure completely. Sodium-glucose cotransporter 2 inhibitor*1) (SGLT2i) is a standard antidiabetic drug and its administration decreases the blood glucose level by inhibiting glucose reabsorption at the renal proximal tubule, thus promoting renal glucose excretion. This insulin-independent glucose disposal effect of SGLT2i, however, decreases the plasma insulin level and increases the glucagon*2) level, leading to a metabolic change similar to that of fasting. Since SGLT2i indeed induces an increase in endogenous glucose production (EGP), the EGP-increasing effect of SGLT2i may unfortunately cancel out its blood glucose‒reducing effect.
Hepatic glucose production (HGP) comprises gluconeogenesis and glycogenolysis, both of which are involved in the HGP-promoting effect of SGLT2i. Because liver glycogen levels are lower in type-2 diabetes and increased gluconeogenesis is responsible for the increased HGP, the effect of SGLT2i on gluconeogenesis plays an important role in its therapeutic impact on type-2 diabetes. Gluconeogenesis is regulated by controlling the gene expression of gluconeogenic enzyme genes such as G6pc*3). Although SGLT2i administration is reported to upregulate the gene expression of G6pc via a plasma glucagon increase and insulin/glucagon ratio decrease, the detailed hepatic gluconeogenic response induced by SGLT2i administration according to insulin resistance has not been elucidated.
[Results]
Scientists of Kanazawa University, in collaboration with scientists from Gunma University, National Center for Global Health and Medicine and Asahi Life Foundation, carried out this research, aiming to elucidate the detailed hepatic gluconeogenic response induced by SGLT2i administration.
First, they generated a hepatic G6pc-reporter mouse model (L-G6pc-GLuc mice) with Gaussia luciferase (GLuc) *4) to elucidate how SGLT2i regulates hepatic gluconeogenesis. Because GLuc is an extracellularly detectable secretory luciferase, its expression, driven by hepatic G6pc promoter*5), can be evaluated by measuring plasma luciferase activity. Thus, L-G6pc-GLuc mice enable evaluation of time-dependent changes in G6pc gene expression in the liver, minimizing variability in results and allowing precise evaluation of gluconeogenesis. Using both lean insulin-sensitive and high-fat diet‒induced insulin-resistant obese L-G6pc-GLuc mice, the scientists evaluated changes over time in gluconeogenesis after single or long-term administration of SGLT2i.
Single administration of SGLT2i to lean mice induced high plasma GLuc activity from 2 hours after administration, indicating the acceleration of the hepatic gluconeogenic response. Using the pancreatic clamp technique*6), they determined whether the decrease in plasma insulin or blood glucose explained the induction of the hepatic gluconeogenic response by single-dose SGLT2i. The results suggested that the SGLT2i-induced decrease in plasma insulin could explain the increase in plasma GLuc activity. On the other hand, it was found that single-dose SGLT2i did not accelerate the hepatic gluconeogenic response in obese mice. These results suggest that the SGLT2i-induced HGP increase in obesity may be caused by mechanisms independent of hepatic gluconeogenic gene induction.
Long-term SGLT2i administration resulted in decreased levels of blood glucose and plasma insulin and increased hepatic gluconeogenic response without changes in plasma glucagon levels in lean mice. On the other hand, in obese mice it decelerated the hepatic gluconeogenic response despite a decrease in plasma insulin level. Long-term SGLT2i administration resulted in the amelioration of hepatic steatosis, which may have also caused the improvement in hepatic insulin resistance.
[Future prospects]
The current study showed that the effects of SGLT2i, a standard antidiabetic drug, depended on the presence or absence of hepatic insulin-resistant. The results obtained are expected to be of much use for treatment of type-2 diabetes patients.
Gaussia luciferase, GLuc, is an extracellularly detectable secretory luciferase and has been used for detection in blood, for example, of various gene expressions. The hepatic G6pc-reporter mouse model, L-G6pc-GLuc mice generated in this study, enabled precise evaluation of the effects of SGLT2i on the hepatic gluconeogenic response through time-dependent changes in G6pc gene expression in the liver of the same mouse by measuring GLuc activity in the blood. The present study thus verified usefulness of this reporter system.
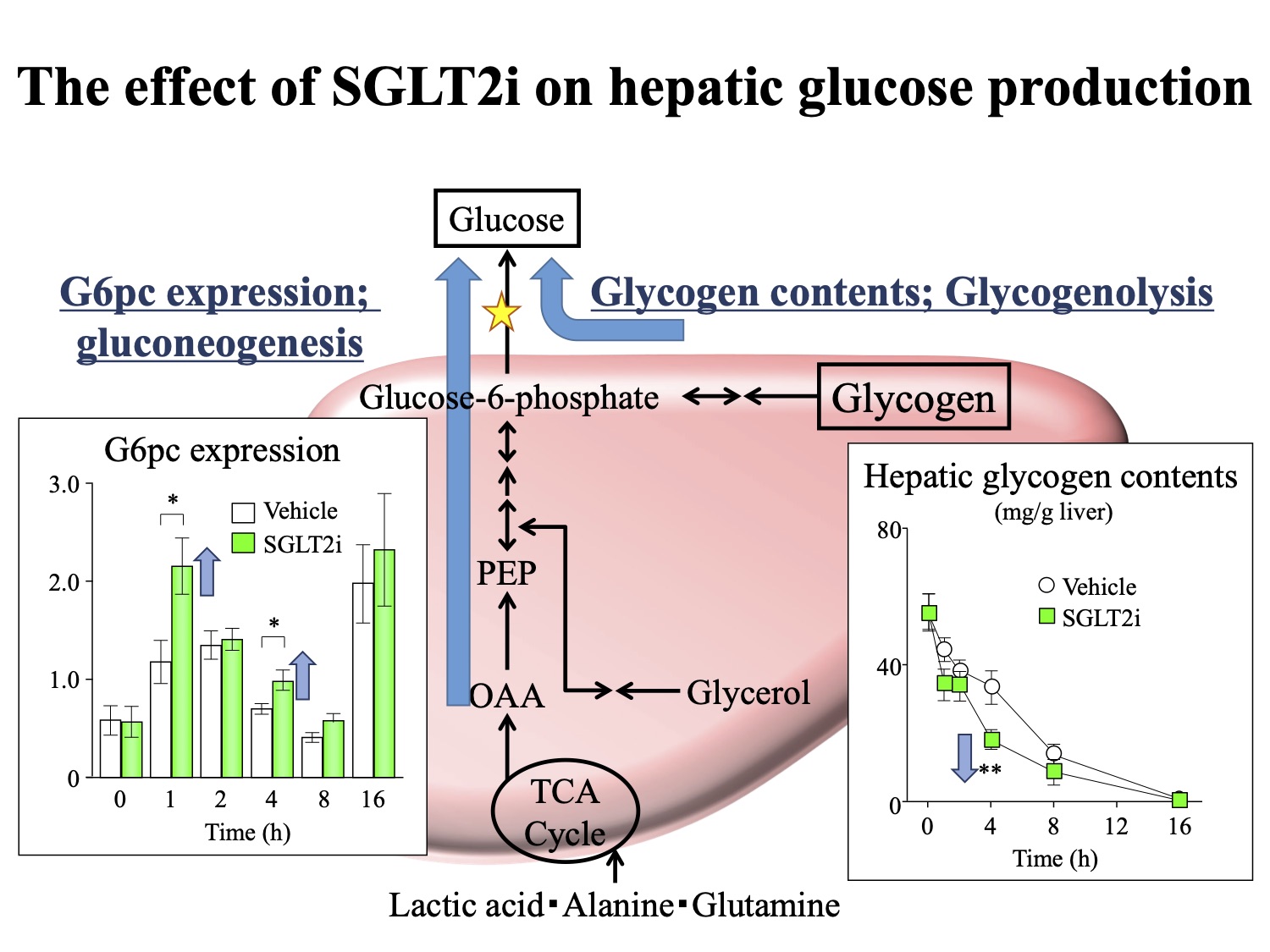
Figure. The effect of SGLT2i on hepatic glucose production (HGP)
HGP comprises gluconeogenesis and glycogenolysis. Gluconeogenesis is regulated by controlling the gene expression of gluconeogenic enzyme genes such as G6pc. SGLT2i increases HGP by affecting both of gluconeogenesis and glycogenolysis.
[Glossary]
*1) Sodium-glucose cotransporter 2 inhibitor (SGLT2i)
Sodium-glucose cotransporter 2 is a glucose transporter protein found in the proximal tubule of the nephron. It contributes to complete renal glucose reabsorption from glomerular filtrate. SGLT2i inhibits sodium-glucose cotransporter 2 activity, contributing to reduction of the blood glucose level and to excretion of glucose in urine. Thus, SGLT2i serves as a medication for type 2 diabetes.
*2) Glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. Glucagon causes the liver to convert stored glycogen into glucose, raising blood glucose level. This effect of glucagon is opposite to that of insulin.
*3) G6pc
G6pc is the gene encoding G6Pase, Glucose-6-phosphatase. G6Pase is a membrane protein of the endoplasmic reticulum that catalyzes the hydrolysis of D-glucose 6-phosphate to D-glucose and phosphate. It is a key enzyme in glucose homeostasis, functioning in gluconeogenesis and glycogenolysis.
*4) Gaussia luciferase (GLuc)
Gaussia princeps, known to display bioluminescence, is a mesopelagic copepod found in temperate and tropical waters worldwide. Gaussia princeps is used in the production of luciferase, Gaussia luciferase, which plays an essential enzymatic role in the bioluminescence emission of Gaussia princeps. Gaussia luciferase is secreted into the body fluid from cells that produce Gaussia luciferase.
*5) G6pc promoter
A promoter is a region of DNA that leads to initiation of transcription of a particular gene. Promoters are located upstream on the DNA near the transcription start sites of genes. G6pc promoter is a promoter that leads to initiation of G6pc transcription.
*6) Pancreatic clamp technique
In order to investigate functions of the pancreas, a catheter is inserted, in case of the mouse, into the cervical vein for perfusion of a solution containing insulin, glucagon, glucose and somatostatin to suppress production and secretion of insulin and glucagon from the pancreas. This allows measurement of the hepatic glucose production with [3-3H]-glucose.
Article
Hepatic Gluconeogenic Response to Single and Long-Term SGLT2 Inhibition in Lean/Obese Male Hepatic G6pc-Reporter Mice
Journal: Endocrinology
Authors: Yuka Inaba, Emi Hashiuchi, Hitoshi Watanabe, Kumi Kimura, Makoto Sato, Masaki Kobayashi, Michihiro Matsumoto, Tadahiro Kitamura, Masato Kasuga, Hiroshi Inoue
Funder
This work was supported by the Japan Society for the Promotion of Science KAKENHI [Grants 17H05499 and 18KT0020 (to H.I.) and 15H05678 (to Y.I.)] and by a research grant from Taisho Pharmaceutical Co., Ltd. (to H.I.).



 PAGE TOP
PAGE TOP