Abstract:
In a recent study published in Autophagy, researchers at Kanazawa University show how abnormalities in a gene called TPR can lead to pediatric brain cancer
Ependymoma is a rare form of brain cancer that implicates children and is often tricky to diagnose. Since effective treatment options can be initiated only after a well-formed diagnosis, there is a dire need among the medical community to identify markers for ependymoma, which in turn, will help oncologists tailor therapy better. Richard Wong’s and Mitsutoshi Nakada’s team at Kanazawa University has now shown how one gene closely linked to ependymoma can help with not just diagnosis, but also treatment options for the condition.
A gene known as TPR shows an elevated presence in 38% of ependymoma cases. Thus, the team first sought out to investigate how an increase in the TPR gene correlated to the development of cancer cells. Each gene present in a cell contains a code for the creation of a specific protein. The TPR gene contains the code for an eponymous protein. Therefore, cancer samples from patients were assessed for the levels of TPR protein. As expected, levels of TPR were abnormally high in these tumor tissues.
The researchers then moved on to investigate whether these abnormal TPR levels could lead to cancer progression. For this purpose, mice were implanted with human ependymoma cancer tissue into their brains. The TPR gene was then deleted in these tissues so that the mice were unable to create the TPR protein. When the tumor tissues were subsequently analyzed, a reduction of cancer growth was seen. The TPR gene was thus vital for the growth of ependymoma tumors.
Deletion of the TPR protein is known to induce a process called autophagy within cells. Autophagy is initiated when a cell is under undue stress and results in the death of damaged cells. The patient tumor samples, with their high levels of TPR protein, showed little or no presence of autophagy. However, autophagy was remarkably high in the mice with TPR depletion. Ependymoma cells were thus spared of autophagic death due to the increased presence of TPR. These damaged cells continued to grow by circumventing the biological systems set up to keep them in check. The high TPR levels were also accompanied by an increase in HSF-1 and MTOR, molecules which are responsible for cell growth and survival.
Finally, the possibility of lowering TPR levels therapeutically to control the cancer was assessed. The mice were given a drug called rapamycin, which inhibits MTOR. The treatment not only led to decreased TPR levels, but also shrank the tumor tissues within their brains.
“Thus, TPR can serve as a potential biomarker, and MTOR inhibition could be an effective therapeutic approach for ependymoma patients”, conclude the researchers. While looking out for increased levels of TPR in patients can help oncologists achieve a more comprehensive diagnosis, reducing TPR levels with the help of drugs can help keep the tumors in check.
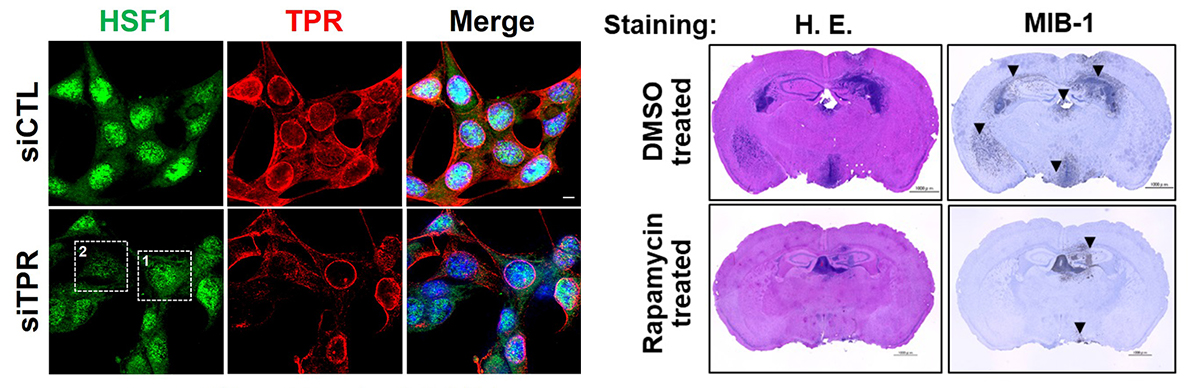
Figure 1.
Deletion of the TPR gene led to lower levels of TPR and its associated protein HSF1 (left; lower panel) whereas rapamycin treatment reduced the tumor cells (dark blue cells) in the brains of mice (right, lower panel)
[Background]
Autophagy: Autophagy, which literally translates to “self-eating” is the self-preservation mechanism of the body to get rid of damaged cells. Autophagy is initiated when an abnormal amount of proteins or toxins build up within a cell, which the cell cannot clear out. Conditions like Alzheimer’s disease and Parkinson’s disease arise when autophagic mechanisms within the cells start malfunctioning. Impaired autophagy is also known to be implicated in driving various forms of cancer.
Article
Nucleoporin TPR (translocated promoter region, nuclear basket protein) upregulation alters MTOR-HSF1 trails and suppresses autophagy induction in ependymoma
Journal: Autophagy
Authors: Firli Rahmah Primula Dewi, Shabierjiang Jiapaer, Akiko Kobayashi, Masaharu Hazawa, Dini Kurnia Ikliptikawati, Hartono, Hemragul Sabit, Mitsutoshi Nakada, and Richard W. Wong.
DOI: 10.1080/15548627.2020.1741318
Funder
This work was supported by the Japan Society for the Promotion of Science [17H05874]; Japan Society for the Promotion of Science [17K08655]; JSPS [B-26293322].



 PAGE TOP
PAGE TOP