Abstract:
Kanazawa University researchers reported that the SUCLA2 gene is frequently involved in the deletion of the tumor suppressor gene RB1 in advanced prostate cancer. RB1 deletion makes cells resistant to hormone therapy but SUCLA2 deletion induces a metabolic weakness. The study showed that thymoquinone selectively killed SUCLA2-deficient prostate cancer cells in vitro and in vivo. The findings highlight a vulnerability of advanced prostate cancer cells that can be targeted by drugs.
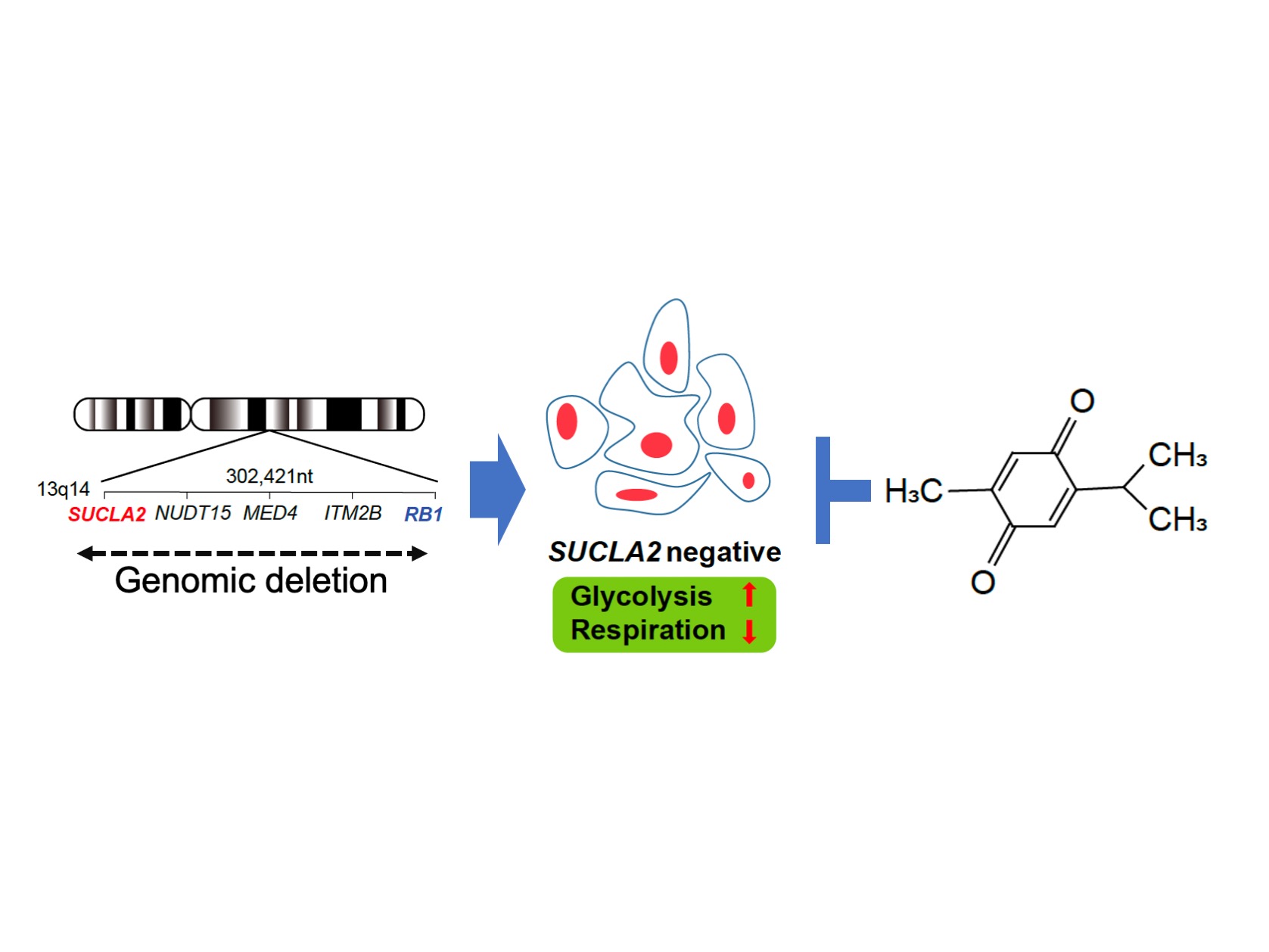
Kanazawa, Japan – The compound thymoquinone (TQ) selectively kills prostate cancer cells at advanced stages, according to a new study published in Oncogene. Led by researchers at Kanazawa University, the study reports that prostate cancer cells with a deletion of the SUCLA2 gene can be therapeutically targeted. SUCLA2-deficient prostate cancers represent a significant fraction of those resistant to hormone therapy or metastatic, and a new therapeutic option for this disease would have immense benefits for patients.
Hormone therapy is often chosen for the treatment of metastatic prostate cancer but nearly half of patients develop resistance to the treatment in as little as 2 years. A mutation in RB1, a tumor suppressor gene that keeps cell growth under control, has been pegged as a particularly strong driver of treatment resistance and predicts poor outcome in patients.
“Mutations in tumor suppressor genes are enough to induce initiation and malignant progression of prostate cancer, but so far we haven’t been able to directly target these mutations with drugs to treat prostate cancer,” says the lead author Susumu Kohno. “We wanted to find a genetic aberration associated with that of a tumor suppressor gene which we could target therapeutically.”
In the genome, SUCLA2 neighbors RB1. An analysis of prostate cancer cells showed that cells with a RB1 deletion were also missing SUCLA2, pairing up the SUCLA2 deletion with the RB1 deletion present in advanced stage prostate cancer. Kohno and colleagues analyzed prostate cancer tissue and found that 11% of cases were missing both SUCLA2 and RB1.
The researchers screened compounds to identify drugs that would selectively kill cells with a SUCLA2 deletion. Out of around 2,000 compounds, TQ emerged as a hit compound. TQ already has known anti-cancer effects and was shown to be safe in a phase I clinical trial. Kohno and colleagues applied the TQ treatment to a mouse model of SUCLA2-deficient prostate cancer and TQ selectively suppressed tumor growth.
“These findings show that TQ treatment could be an effective therapy for treating prostate cancer cells that harbor SUCLA2 deficiency” says the senior author Chiaki Takahashi.
In a search of genetic databases from patients with prostate cancer, the researchers found that the frequency of SUCLA2 loss was almost perfectly aligned with RB1 loss at every disease stage—meaning the SUCLA2 deletion could identify people with prostate cancer needing advanced therapy.
Finding this drug-targetable vulnerability opens a crack in the barrier of treatment resistance for prostate cancer. More work needs to be done to improve efficacy of TQ and identify patients that would benefit from this type of treatment, but the compound provides a promising route for new treatment options for advanced prostate cancer.
Graphical abstract
SUCLA2 gene is frequently involved in the deletion of RB1 gene region, which occurs in 10 – 30 % of advanced prostate cancers. SUCLA2 deletion gives rise to a metabolic vulnerability. By screening chemical compounds, thymoquinone appeared to selectively kill SUCLA2-deficeint prostate cancer cells.
Article
Pharmacologically targetable vulnerability in prostate cancer carrying RB1-SUCLA2 deletion
Journal: Oncogene
Authors: Susumu Kohno, Paing Linn, Naoko Nagatani, Yoshihiro Watanabe, Sharad Kumar, Tomoyoshi Soga, Chiaki Takahashi
DOI: 10.1038/s41388-020-1381-6
Funder
This study was supported by Funding Program for Next Generation World-Leading Researchers (LS049) from cabinet office of Japan, Grant-in-Aid for Scientific Research (17H03576, 17K19586, 19K22555, 17K14992, 20K07612) from MEXT, Project for Cancer Research and Therapeutic (P-CREATE) (19cm0106164h0001) from Japan Agency for Medical Research and Development (AMED), TaNeDS (C1010568) and a collaborative research fund (C1010818) from Daiichi-Sankyo Co. Ltd., and a Senior Principal Research Fellowship from the National Health & Medical Research Council of Australia (GNT 1103006).



 PAGE TOP
PAGE TOP