Abstract:
The research group of Professor Rikinari Hanayama including doctoral students of the Graduate School of Medical Sciences at Kanazawa University, Tuan Duc Nguyen and Yuji Miyatake (currently a research fellow at The Institute of Medical Sciences, Tokai University) and two others identified a protein that causes adipose tissue loss in cancer-associated cachexia*1).
Cancer-associated cachexia is characterized by a reduction of body weight and atrophy accompanied with progression of cancer and is found in about 80% of patients with progressive cancer. Since cancer-associated cachexia causes various problems such as difficulty in walking and diminished effectiveness of anti-cancer drug treatment, elucidation of the mechanism and development of prevention methods are needed.
In this study, the research group revealed that a protein called proliferin-1*2) released by cancer cells acts on adipocytes to control the inhibition of adipogenesis and the promotion of lipolysis. First, by adding culture supernatants from various cancer cells to pre-adipocytes, the culture supernatants that inhibited adipogenesis were selected (Figure. 1). Next, by fractionating and analyzing the components of the culture supernatants, proliferin-1 was identified as the protein that caused inhibition of adipogenesis. In fact, the addition of a recombinant*3) proliferin-1 to pre-adipocytes inhibited adipogenesis (Figure. 2). In addition, when proliferin-1 was added to mature adipocytes, it accelerated lipolysis of stored fat. These effects caused adipose tissue loss upon administration of proliferin-1 to mice. Furthermore, it was revealed that when cancer cells were transplanted into normal mice, adipose tissue loss was induced due to cancer-associated cachexia, but that in the case of transplantation of cancer cells deficient in proliferin-1, it was found that the loss was suppressed.
Thus, this study revealed that proliferin-1 released by cancer cells induced adipose tissue loss in cancer-associated cachexia, suggesting a possibility of improvement of cancer-associated cachexia by inhibiting proliferin-1 function. It is expected that further research and development of proliferin-1 as a therapeutic target will be explored for improvement of QOL*4) and prognosis of cancer patients.
The research results were published as an “Accepted Article” in the journal “International Journal of Cancer” of the Union for International Cancer Control (UICC) on November 30, 2020.
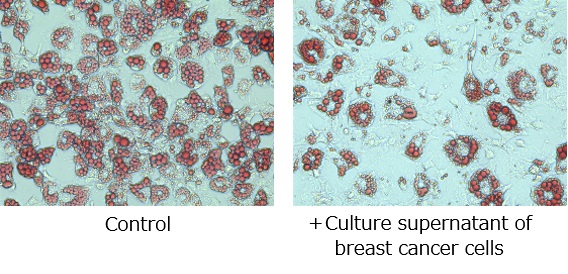
Figure1. Inhibition of adipogenesis by culture supernatant of cancer cells.
Fat stored in pre-adipocytes was detected with a red dye. Adipogenesis was significantly inhibited by the addition of culture supernatant of breast cancer cells as compared with the control.
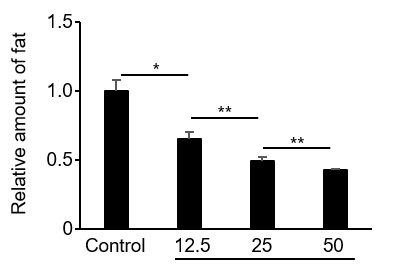
Figure2. Inhibition of adipogenesis by proliferin-1.
The amount of stored fat of pre-adipocytes was measured 5 days after addition of recombinant proliferin-1. Relative ratio with the control without any addition is shown. (*p < 0.01; **p < 0.05)
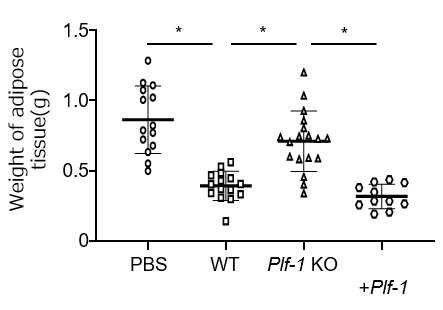
Figure3. Reduction of adipose tissue loss in cancer cells expressing proliferin-1.
Transplantation of wild-type (WT) cancer cells into mice reduced the weight of gonadal adipose tissue compared to the non-transplanted control group (PBS). On the other hand, transplantation of cancer cells deficient in proliferin-1 (Plf-1 KO) suppressed the reduction. However, re-expression of proliferin-1 (Plf-1 KO + Plf-1) re-induced adipose tissue weight reduction. (*p < 0.01)
[Glossary]
*1) Cancer-associated cachexia
A syndrome characterized by loss of adipose tissue and skeletal muscle and body weight is lost due to the morbidity of cancer, which is not fully reversed by normal nutritional support.
*2) Proliferin-1
A protein identified as a placental hormone. Functions such as, e.g. in angiogenesis have been reported.
*3) Recombinant protein
A protein produced in a large quantity by recombinant DNA technology.
*4) QOL
Acronym of Quality of Life, meaning the physical, mental and social quality of life of the patient.
Article
Tumor‐secreted proliferin‐1 regulates adipogenesis and lipolysis in cachexia
Journal: International Journal of Cancer
Authors: Tuan Duc Nguyen, Yuji Miyatake, Takeshi Yoshida, Hironori Kawahara, Rikinari Hanayama
DOI: 10.1002/ijc.33418
Funder
This work was supported by the Core Research for Evolutional Science and Technology (CREST) from Japan Science and Technology Agency (JST) (JPMJCR18H4), Grants‐in‐Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (19H03433), and the World Premier International Research Center Initiative (WPI), MEXT, Japan.



 PAGE TOP
PAGE TOP