Abstract:
Researchers at Kanazawa University report in Communications Chemistry that a molecule known as pillar[6]arene can form a host–guest compound with a cancer-associated metabolite. The phenomenon can be used to efficiently detect the metabolite in crude biological samples, which is important for preventing and treating metabolic syndrome and associated pathologies.
Metabolites are organic molecules that take part in or are created during the biochemical reactions constantly taking place in an organism. For the human body, more than 110,000 metabolites have been identified. Metabolites play a role in metabolic syndrome, which is the situation in which several medical conditions occur simultaneously; the conditions include obesity, high blood pressure and high blood sugar. Metabolic syndrome is associated with a higher risk of developing cardiovascular disease, type-2 diabetes and different kinds of cancer. The presence of certain metabolites can be an indicator for particular pathological conditions related to metabolic syndrome. Efficiently measuring and monitoring the presence is therefore important for early diagnosis. Now, Tomoki Ogoshi*, Atsushi Hirao*, and Masaya Ueno (*correspondence authors) from Kanazawa University and colleagues have developed a biosensor for a low-molecular-weight metabolite known as 1-MNA. The sensor relies on the physicochemical properties of pillar[6]arene, a channel-like molecule.
The researchers investigated the metabolite 1-MNA (1-methylnicotinamide), recently discovered to be present in higher levels in aggressive cancer cell lines. These cancers have increased NNMT (nicotinamide N-methyltransferase) activity in which 1-MNA is a byproduct. Detecting 1-MNA could be therefore crucial for the timely diagnosis and treatment of such cancers.
Ogoshi and colleagues hypothesized that pillar[n]arenes could be used as biosensors for metabolites like 1-MNA. Pillar[n]arenes are pillar-shaped macrocyclic compounds with a polygonal cross-section (pentagonal and hexagonal for n = 5 and 6, respectively). The researchers found that pillar[6]arene (P6A) forms host–guest a complex with 1-MNA; the metabolite can bind to it because the hexagonal cavity inside P6A offers just the right environment for doing so. They also found that when 1-MNA is bound to P6A, the fluorescent response of the latter significantly decreases — an effect that can be exploited as an indicator for the presence or absence of 1-MNA (strong or weak fluorescent response, respectively).
Importantly, the scientists could show that the P6A fluorescence detection mechanism works for biological samples. Specifically, they were able to detect 1-MNA in urine, albeit with a low sensitivity. Ogoshi and colleagues conclude that additional experiments “will help to improve the sensitivity and specificity of the biosensors”, and that their work “should contribute to the development of low-cost, easy, and rapid methods for the detection of human metabolites for diagnosis”.
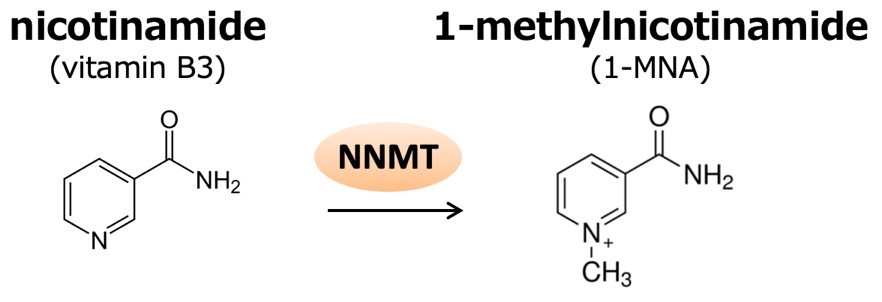
Figure 1.
NNMT catalyzes the methylation of nicotinamide to produce 1-methylnicotinamide (1-MNA). 1-MNA can be further oxidized by aldehyde oxidase into, N1-methyl-2- pyridone-5-carboxamide (2py) or N1-methyl-4-pyridone-3-carboxamide (4py), and all three metabolites are excreted in the urine.
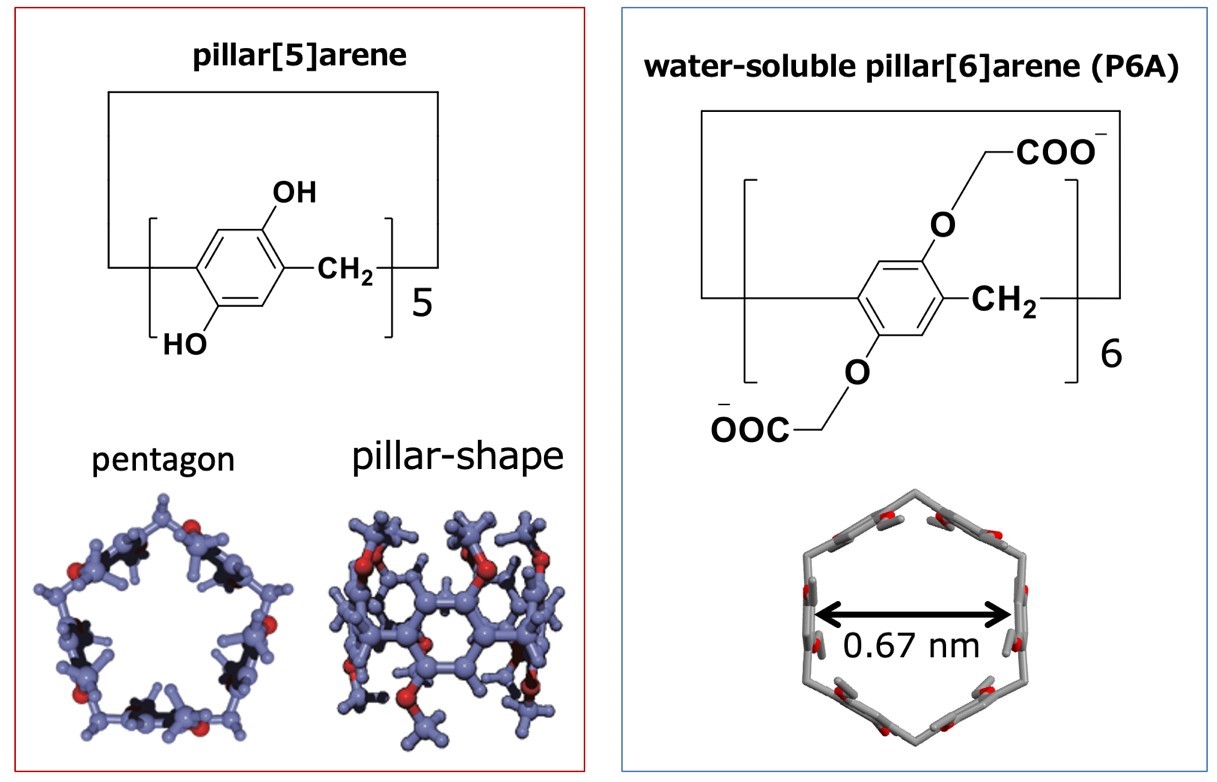
Figure 2.
Pillar[n]arenes, which were first reported by Ogoshi’s group in 2008, are a family of pillar- shaped macrocyclic compounds that contain a para-bridge connection between the 1,4-dialkoxybenzene units. They have an electron-rich cavity that has host–guest properties, and it is known that pillar[n]arenes form stable complexes with cations. A water-soluble pillar[6] arene (P6A) is carrying 12 carboxylate anions. The diameter across the cavity of P6A is approximately 0.67 nm.
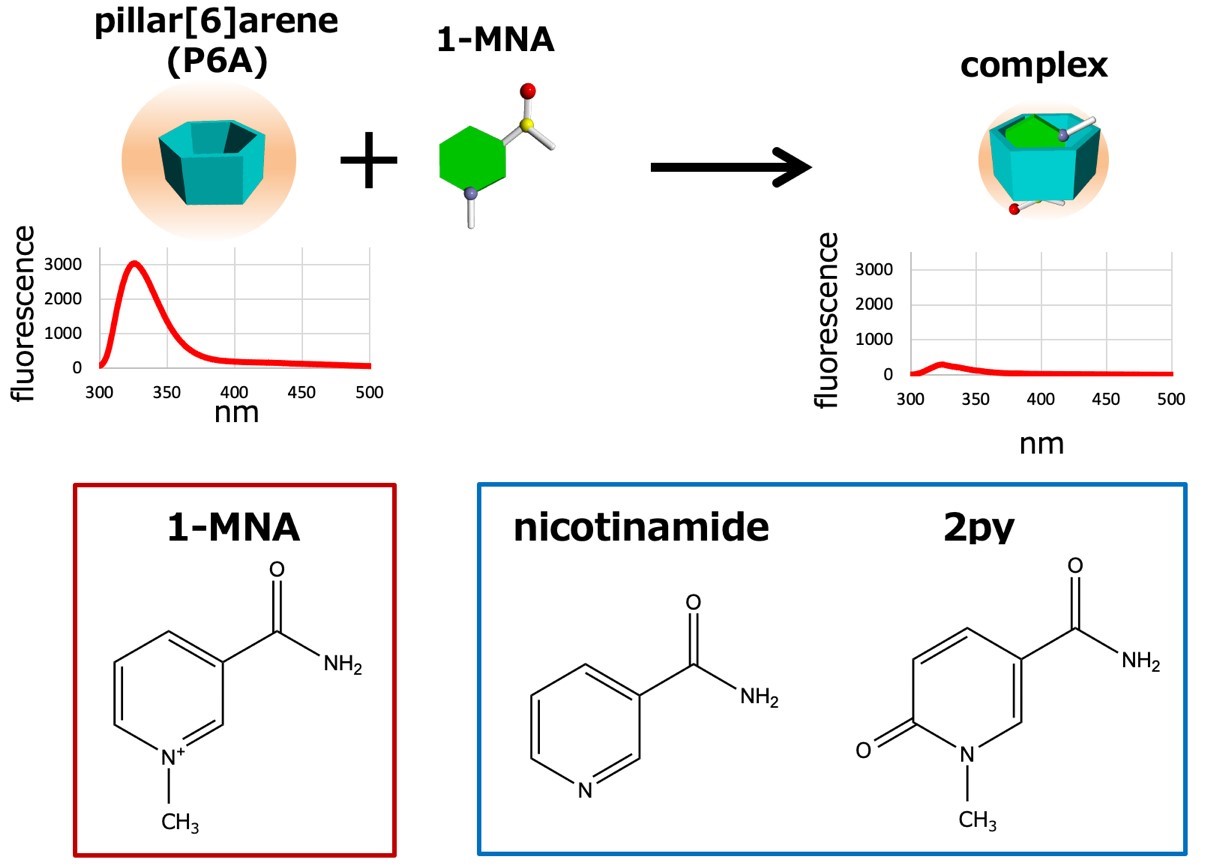
Figure 3.
Upon addition of 1-MNA, the fluorescence intensity of P6A dramatically decreased because of photoinduced electron transfer. P6A also has high selectivity: P6A do not form host–guest complexes with nicotinamide and 2py.
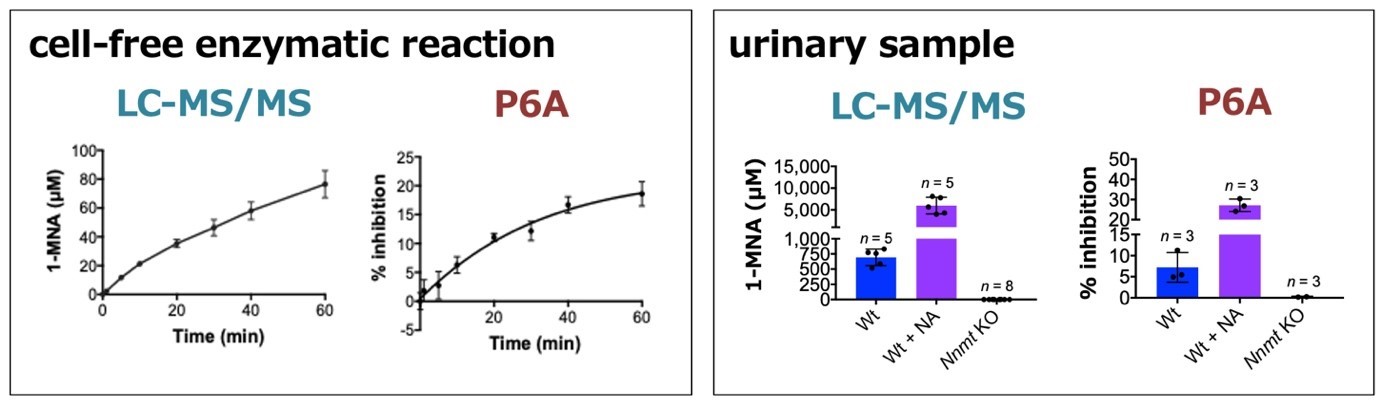
Figure 4.
(left) In a cell-free enzymatic reaction, 1-MNA is produced by NNMT in a time-dependent manner. P6A is used to monitor production of 1-MNA in this system. (right) P6A is also useful to detect 1- MNA in a crude biological sample. In animals, the concentration of urinary 1-MNA is highly dependent on the activity of NNMT and the total uptake of nicotinamide from foods. Therefore, intake of nicotinamide leads to an increase in the urinary excretion of 1- MNA in wild-type mice, while no 1-MNA excretion is detected in the Nnmt deficient mice. The fluorescence intensity of P6A is significantly quenched by wild-type derived urine samples. In addition, the fluorescence intensity was quenched by 1-MNA in a dose-dependent manner.
(LC-MS/MS: quantification data using LC-MS/MS, P6A: data from fluorescence spectra with P6A, Wt: wild-type mice, Wt + NA: nicotinamide treatment group, Nnmt KO: NNMT deficient mice)
[Background]
Metabolic syndrome
Metabolic syndrome refers to the combination of diabetes, high blood pressure (hypertension) and obesity. Patients with metabolic syndrome are at a greater risk for developing certain cardiovascular diseases as well as different types of cancer. Metabolic syndrome is often associated with being overweight and a lack of physical activity, and is also linked to insulin resistance (a key feature of type-2 diabetes).
Now, Tomoki Ogoshi from Kanazawa University and colleagues have developed a biosensor for a metabolite known as 1-MNA. Being able to efficiently detect metabolites associated with certain pathologies is an important step forward towards the development of treatments for pathologies associated with metabolic syndrome.
Pillar[n]arenes
Pillar[n]arenes, collectively named pillararenes (and sometimes pillarenes), are cyclic organic molecules consisting of n so-called hydroquinone units, which can be substituted. Hydroquinone, also known as quinol, has the chemical formula C6H4(OH)2. It consists of a benzene ring with two hydroxyl (OH) groups bound to it at opposite sides of the benzene hexagon.
The first pillararene was synthesized in 2008 by Tomoki Ogoshi and colleagues from Kanazawa University. The name pillararene was chosen since the molecules are cylindrical (pillar-like) in shape and composed of aromatic moieties (arenes).
Now, Ogoshi, Hirao and colleagues have shown that pillar[6]arene (n = 6) can be used as biosensors for the metabolite 1-MNA — an important result given that detecting low-molecular-weight metabolites is challenging.
Article
Pillar[6]arene acts as a biosensor for quantitative detection of a vitamin metabolite in crude biological samples
Journal: Communications Chemistry
Authors: Masaya Ueno, Takuya Tomita, Hiroshi Arakawa, Takahiro Kakuta, Tada-aki Yamagishi, Jumpei Terakawa, Takiko Daikoku, Shin-ichi Horike, Sha Si, Kenta Kurayoshi, Chiaki Ito, Atsuko Kasahara, Yuko Tadokoro, Masahiko Kobayashi, Tsutomu Fukuwatari, Ikumi Tamai, Atsushi Hirao, and Tomoki Ogoshi
DOI: 10.1038/s42004-020-00430-w
Funder
Grant-in-Aid for Scientific Research, MEXT(17K09919, 19H01033, 19H00909); Grant-in-Aid for Project for Cancer Research and Therapeutic Evolution (P-CREATE)(19CM0106104H0004), AMED; JST CREST(JPMJCR18R3); WPI-NanoLSI Transdisciplinary Research Grant from Kanazawa University; World Premier International Research Center Initiative (WPI)



 PAGE TOP
PAGE TOP