Abstract:Researchers at Kanazawa University have optimized a base-catalyzed three-component reaction between nitroalkanes, acrylamides, and aldehydes that proceeds through a domino Michael/aldol process. Mechanistic studies enabled them to identify a key intermediate and confirm the role of the solvent in promoting the reaction. The β’-hydroxy-γ-nitro amide products are used as reagents for syntheses of antimicrobial agents. Their environmentally benign production has paved the way for new green synthetic strategies involving multiple components.
Kanazawa, Japan – Environmental concerns have shifted the focus of organic chemists to developing simple, environmentally friendly ways to synthesize and study compounds that are relevant to human health. Now, researchers from Kanazawa University have achieved exactly that using a catalytic domino reaction as an efficient synthetic method for producing structurally complex molecules from three simple components.
As published in Green Chemistry, the team optimized the base-catalyzed synthesis of various types of β’-hydroxy-γ-nitro amide compounds and identified a key intermediate along the reaction pathway that shed light on the mechanism and the wider implications for chemical synthesis.
Bioactive compounds are synthesized from β’-hydroxy-γ-nitro amides, but they are difficult to synthesize because they contain multiple chemical functional groups (OH, NO2, N-C=O). The researchers aimed to construct these complex molecules by combining three compounds, each with one of the critical groups; specifically, nitroalkanes containing NO2, acrylamides containing N-C=O, and aldehydes containing HC=O, which formed OH groups during the reaction. To link these three molecules, two organic synthesis tools were employed. This domino reaction technique had been used before, but never for catalyzed the three-component systems.
“We knew the experimental conditions would influence the reaction outcome,” explains Shunya Morita, a senior author of the study. “After screening various solvents and catalysts, we were happy to find that the combination of DMSO [dimethyl sulfoxide solvent] and KOH [potassium hydroxide catalyst] gave the best results because this meant that the reaction could be performed in an environmentally friendly way.”
They also explored the scope of the reaction using variations of the three initial components and confirmed that numerous combinations could be accommodated, thus demonstrating the versatility of the system. To better understand how the domino reaction occurred, they performed detailed mechanistic studies.
“We were able to monitor the reaction in real time and see the emergence and consumption of the key intermediate, plus the orientation of certain bonds in the final products” says the principal investigator, Jun-ichi Matsuo. These experiments confirmed that the first step of the process linked two of the three initial components, thus generating a short-lived intermediate, which promoted the second step to link the third component. “The DMSO solvent played a key role in supporting the properties of the intermediate that allowed the rest of the reaction to proceed as designed,” Matsuo adds.
The ability to use environmentally friendly conditions to access bioactive molecules is crucial for implementing sustainable organic synthetic methods, and the expanded understanding of domino reactions could be a game-changer for the development of other multi-component reactions.
Figure1
Only a catalytic amount of KOH catalyzed three-component domino reactions to afford chemically and biologically important compounds in an environmentally friendly single manner.
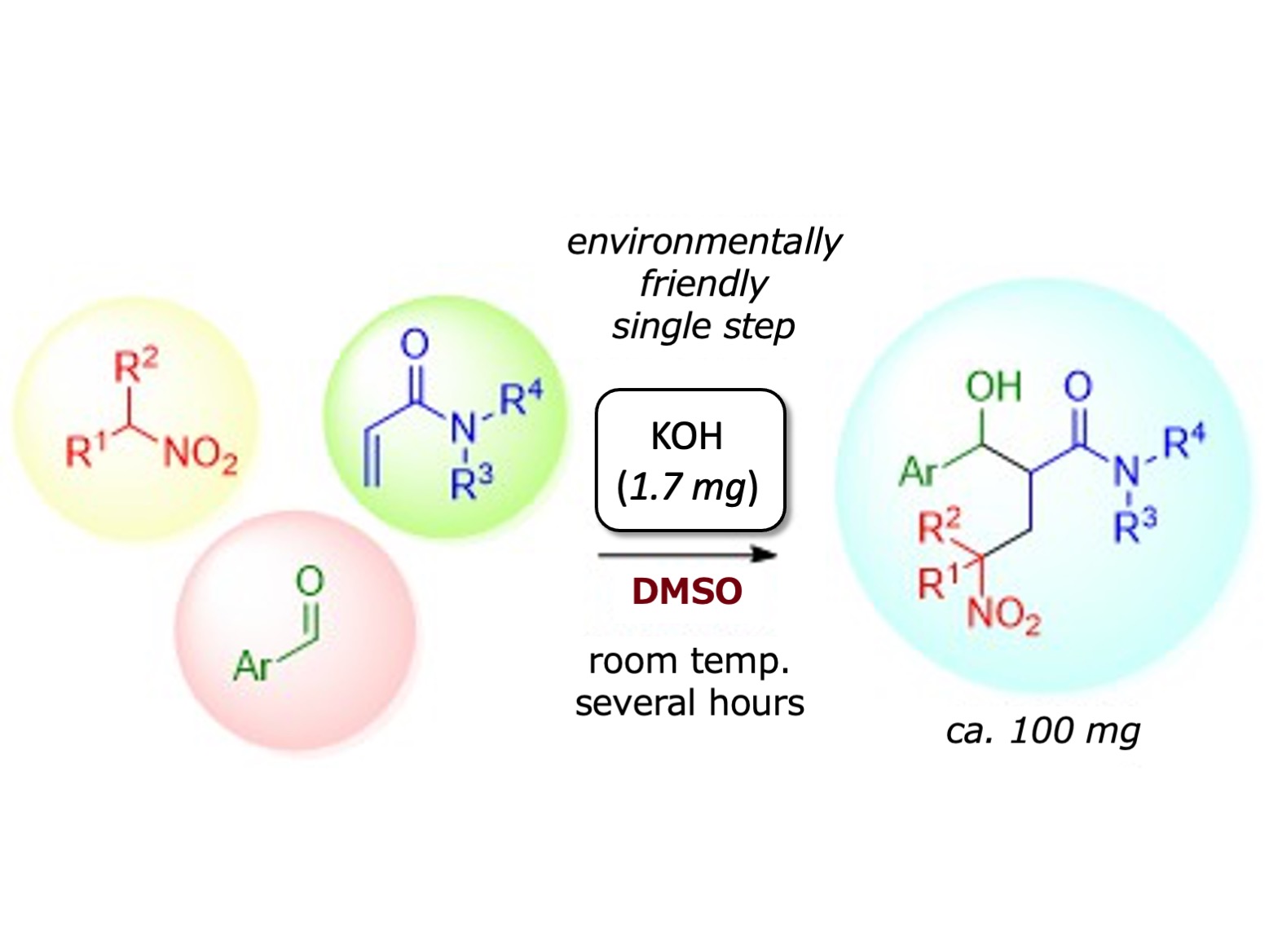
Figure2
Environmentally friendly three-component domino aldol/Michael reactions of nitroalkanes, acrylamides, and aldehydes were catalyzed with less than 10 mol% of KOH in DMSO.
[Funder]
JSPS (KAKENHI Grant Number JP19K05473)
[Article]
Title:Catalytic intermolecular aldol reactions of transient amide enolates in domino Michael/aldol reactions of nitroalkanes, acrylamides, and aldehydes,
Journal:Green Chemistry
Authors:Shunya Morita, Tomoyuki Yoshimura, and Junichi Matsuo



 PAGE TOP
PAGE TOP