Abstract: Researchers at Kanazawa University report in Science Advances a new method for distinguishing between enantiomers, molecules that are mirror images of each other. The procedure, relevant for the pharmaceutical industry, involves the chemical reaction of target enantiomers with color indicator compounds consisting of one-handed helical polymers, leading to solutions showing different colors in specific solvents between the enantiomers.
Enantiomers are molecules that are each other’s mirror image — like one’s left and right hand. They are said to be chiral, chirality being the term for ‘displaying handedness’. Although a pair of enantiomers have totally the same chemical and physical properties, they often exhibit different physiological activity towards biological molecules. Being able to distinguish between enantiomers and detect chirality is important for pharmaceutical purposes — often, only one of two enantiomers acts as a drug. Now, Katsuhiro Maeda from Kanazawa University and colleagues have found a new method for determining the chirality of amines (organic molecules featuring amino groups (-NH2)). The approach is based on reactions leading to solutions with different colors depending on the enantiomer present.
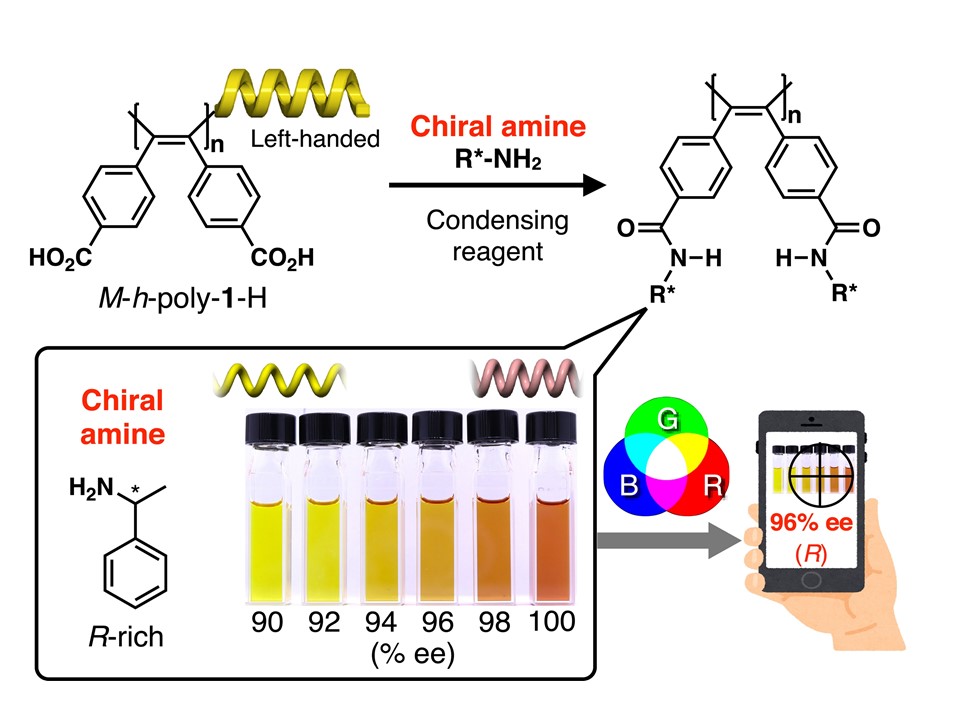
The method of Maeda and colleagues involves the use of special organic ‘color indicator’ molecules consisting of one-handed helical poly(diphenylacetylene)s possessing carboxy groups in the side chains (M–h-poly-1-H and P–h-poly-1-H), which are chiral themselves because they have so-called one-handed (right- or left-handed) helical structures (the ‘M’ and the ‘P’ refer to the left- and right-handed configurations, respectively). The scientists serendipitously discovered that a pair of enantiomers of particular chiral amines, when reacting with M–h-poly-1-H using a condensing reagent, displayed completely different colors in particular solvents (for example, in tetrahydrofuran-acetone, yellow and red, respectively) depending on their chirality, thereby enabling easy naked-eye differentiation between the enantiomers.
The researchers tested a whole set of other amines, as well as other nitrogen-containing organic molecules (specifically, amino alcohols and amino esters), also showing distinct colorings detectable by the naked eye. Some solutions had to be cooled down to -60 °C, however.
Computer simulations of the compounds together with various experimental analysis provided insights into the molecular mechanisms at play. They showed that for one enantiomer, intramolecular hydrogen bonding (attraction between hydrogen atoms within a molecule) does not happen, resulting in a stretched helical structure and a yellow solution, whereas it does for the other enantiomer, causing the molecular helix to contract, resulting in a red-colored solution.
The scientists used their finding to develop a procedure for obtaining the so-called enantiomeric excess (ee) of a mixture of chiral molecules, a measure of the enantiomeric ‘purity’: an ee of 0% means an equal amount of left- and right-handed molecules, whereas an ee of 100% corresponds to the situation of only one type of enantiomer being present. For this, they quantified the color measurement by recording absorption spectra or by digital photography by converting to RGB (red, green, blue) values; these depend on a mixture’s ee. Low-error determinations could be made that were in excellent agreement with measurements obtained by the current standard technique (called high-performance liquid chromatography).
Maeda and colleagues reckon that they can design other indicator molecules and expand the method. Quoting the researchers: “This should be applicable to the on-site, naked-eye determination of ee of various functional molecules and biologically relevant compounds”.
Figure
Organic ‘color indicator’ compounds consisting of one-handed helical poly(diphenylacetylene)s possessing carboxy groups in the side chains (M–h-poly-1-H) for distinguishing between enantiomers of chiral amines and for determining their enantiomeric excess.
[Article]
Title: Helical springs as a color indicator for determining chirality and enantiomeric excess
Journal: Science Advances
Authors: Katsuhiro Maeda, Daisuke Hirose, Mai Nozaki, Yoichi Shimizu, Taro Mori, Kentaro Yamanaka, Koji Ogino, Tatsuya Nishimura, Tsuyoshi Taniguchi, Munetsugu Moro, and Eiji Yashima.



 PAGE TOP
PAGE TOP