Abstract: Researchers at Kanazawa University evaluated the application of a probe containing the diagnostic radiometal gallium-68 and a probe containing the therapeutic radiohalogen astatine-211. Both probes showed similar biodistribution and accumulation within tumor-bearing mice, illustrating the potential for radionuclide-labeled probes with different chemical characteristics to be used in combined diagnostic and therapeutic cancer treatments.
Kanazawa, Japan – An approach called radiotheranostics involves a combination of a different kind for the treatment of cancer – namely, the combination of therapeutic and diagnostic techniques using radionuclides, chemical elements with unstable nuclei that emit radiation. Now, researchers in Japan have investigated a new strategy in radiotheranostics that involves another combination: metals and halogens.
In a study published in Molecular Pharmaceutics, researchers from Kanazawa University have demonstrated the feasibility of the dual application of diagnostic and therapeutic probes containing the radiometal gallium-68 (68-Ga) and the radiohalogen astatine-211 (211-At) for potential cancer treatment.
In radiotheranostics, imaging techniques like positron emission topography (PET), which involves the use of radionuclide-labeled probes, are used to locate tumor cells in the body, and a radiolabeled drug is targeted to these tumor cell sites for treatment. Typically, the combination of imaging and therapeutic radionuclides is restricted to the use of elements with similar characteristics – for example, a therapeutic probe containing the radionuclide 211-At may be combined with a diagnostic probe containing iodine, because both elements are halogens. However, the combination of more diverse elements for radiotheranostic treatment would increase the versatility of this approach and allow for more personalized cancer treatment.
“We wanted to evaluate the use of radiotheranostic probes with different characteristics,” says lead author Kazuma Ogawa. “Specifically, we investigated the use of a probe containing the metal 68-Ga, which is used in PET imaging to identify tumors, and a probe containing the halogen 211-At, which is used in targeted alpha therapy to treat tumors.”
The researchers designed and synthesized three radionuclide-labeled probes each with two radiolabeling sites for the radiometal and radiohalogen. U-87 MG glioblastoma cells were used to evaluate the binding characteristics and stability of the probes, while tumor-bearing mice were used to evaluate the biodistribution, or where the probes traveled and accumulated within the body.
“We were pleased to see that the Ga-based probe and the At-based probe showed similar biodistribution and high accumulation in the mouse tumors,” says senior author Seigo Kinuya.
The results indicate the potential for the use of multiradionuclide probes in radiotheranostic treatment. A greater diversity of probe combinations, such as the combination of a radiometal and radiohalogen evaluated here, may facilitate more personalized cancer treatments in the future.
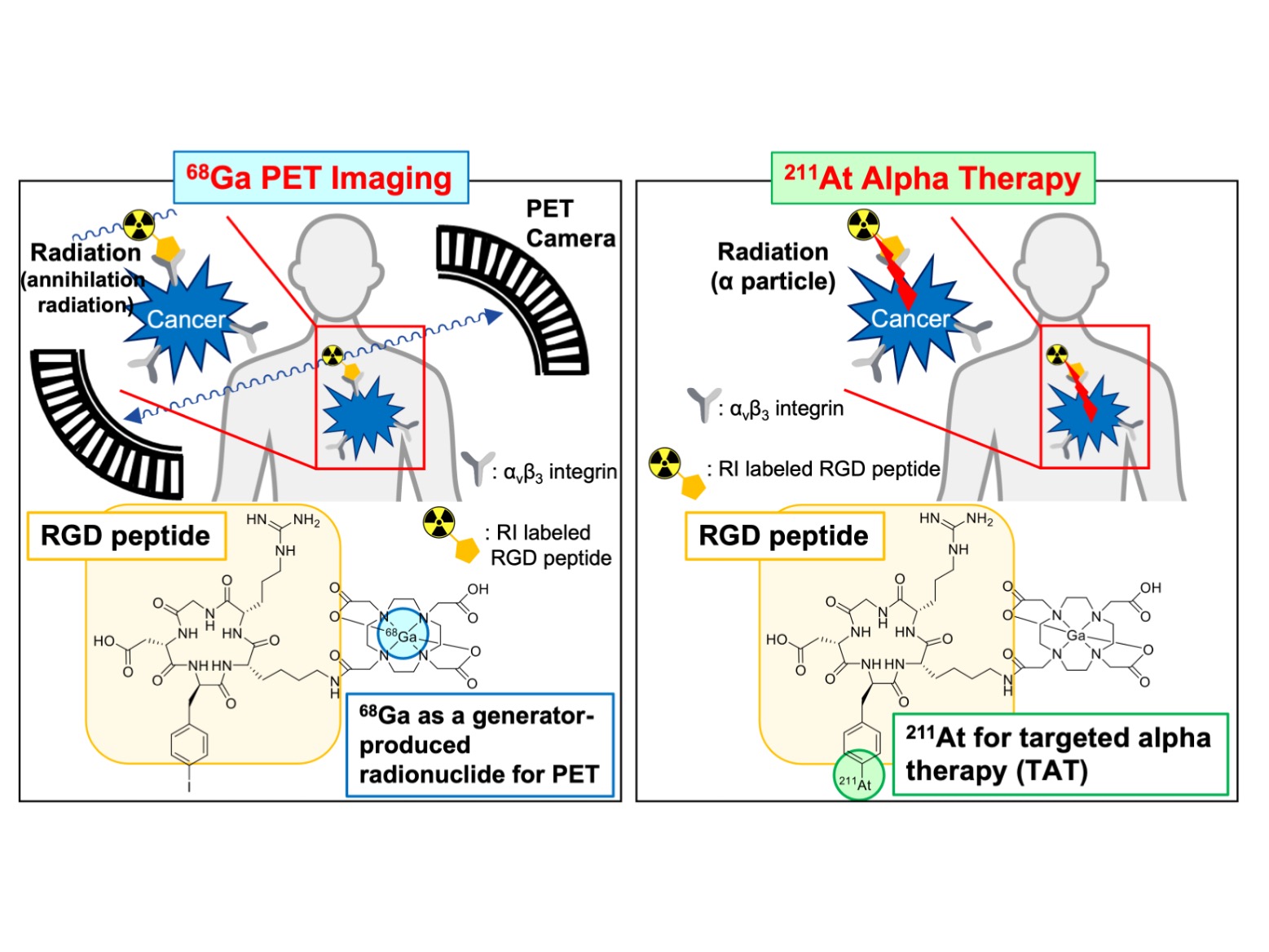
Figure1
The word “theranostics”, a portmanteau of the words “therapeutics” and “diagnostics”. Theranostics with radioisotopes is called “radiotheranostics”, which means the combination of imaging in nuclear medicine with radionuclide therapy. Probes for radiotheranostics could be produced by introducing radionuclides with similar chemical characteristics into the same precursors. Here, K. Ogawa et al. hypothesized that probes for radiotheranostics combined with multiradionuclides, such as 68Ga and 211At, have useful clinical applications. New radiolabeled RGD peptide probes were synthesized via a molecular design approach, with two labeling sites for metal and halogen. These probes in radiotheranostics with multiradionuclides, such as a radiometal and a radiohalogen could contribute to a personalized medicine regimen.
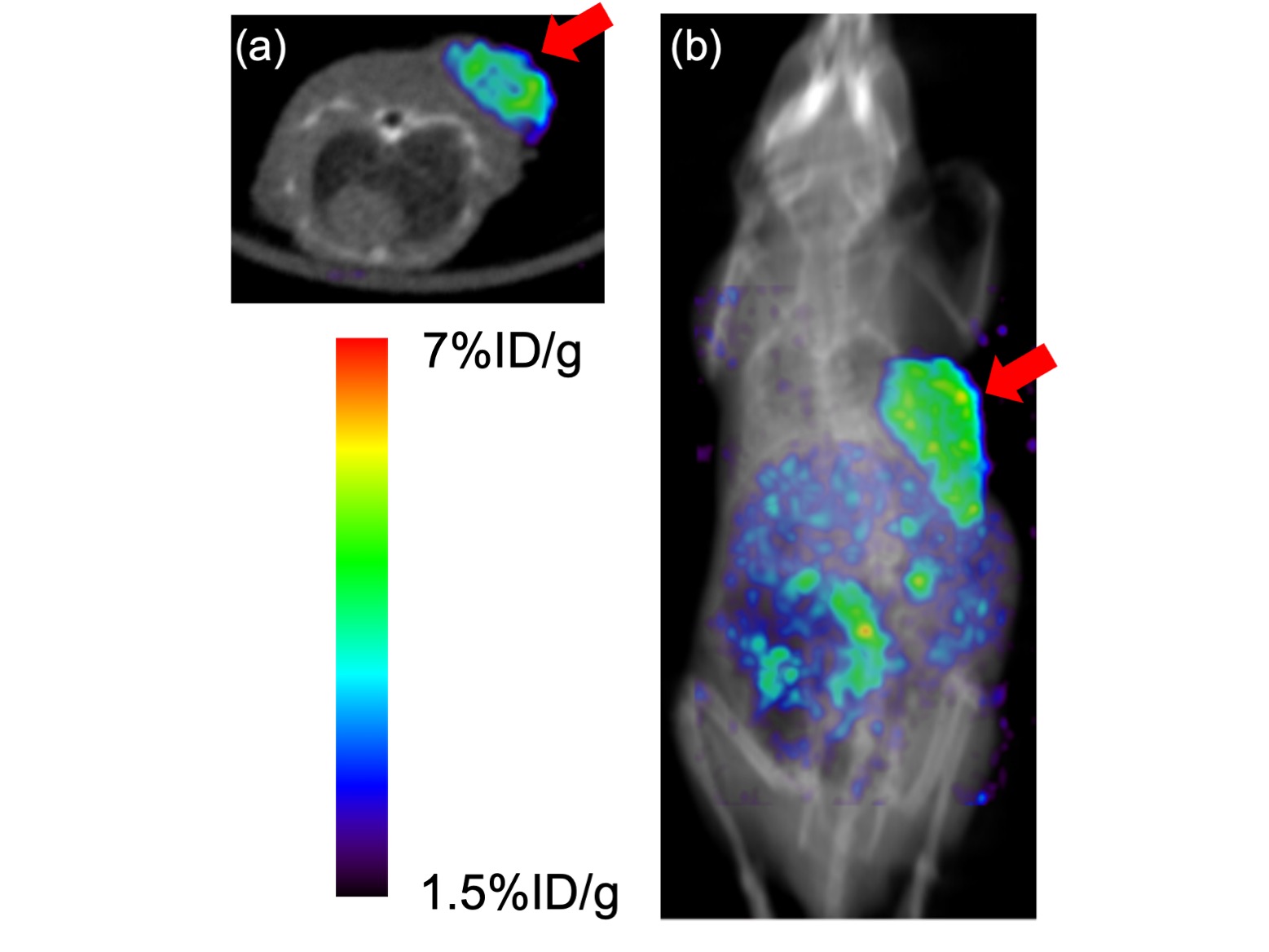
Figure2
Representative single photon emission computed tomography (SPECT)/CT images. (a) axial SPECT/CT fusion image and (b) coronal MIP SPECT/CT fusion image of a U-87 MG tumor-bearing mouse at 4 h postinjection of 67Ga-labeled RGD peptide. 67Ga was used as an alternative radionuclide to 68Ga. Arrows indicate the site where U-87 MG cells were inoculated.
[Funder]
This work was supported in part by Grants-in-Aid for Scientific Research (21H02867) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, Mitani Foundation for Research and Development, Pancreas Research Foundation of Japan, Kanazawa University SAKIGAKE project 2020, and Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science.
[Article]
Title: 68Ga- and 211At-Labeled RGD Peptides for Radiotheranostics with Multiradionuclides
Journal: Molecular Pharmaceutics
Authors: Kazuma Ogawa, Hiroaki Echigo, Kenji Mishiro, Saki Hirata, Kohshin Washiyama, Yoji Kitamura, Kazuhiro Takahashi, Kazuhiro Shiba, and Seigo Kinuya



 PAGE TOP
PAGE TOP