Researchers at Kanazawa University report in JACS Au how they have developed operando scanning ion conductance microscopy to allow simultaneous measurements of changes in the anode surface topography of a lithium ion battery during use, as well as the varying ion concentration with depth. Combining both types of information should help researchers evaluate the correlation between the two to design better batteries.
———————————————————————————————————————————-
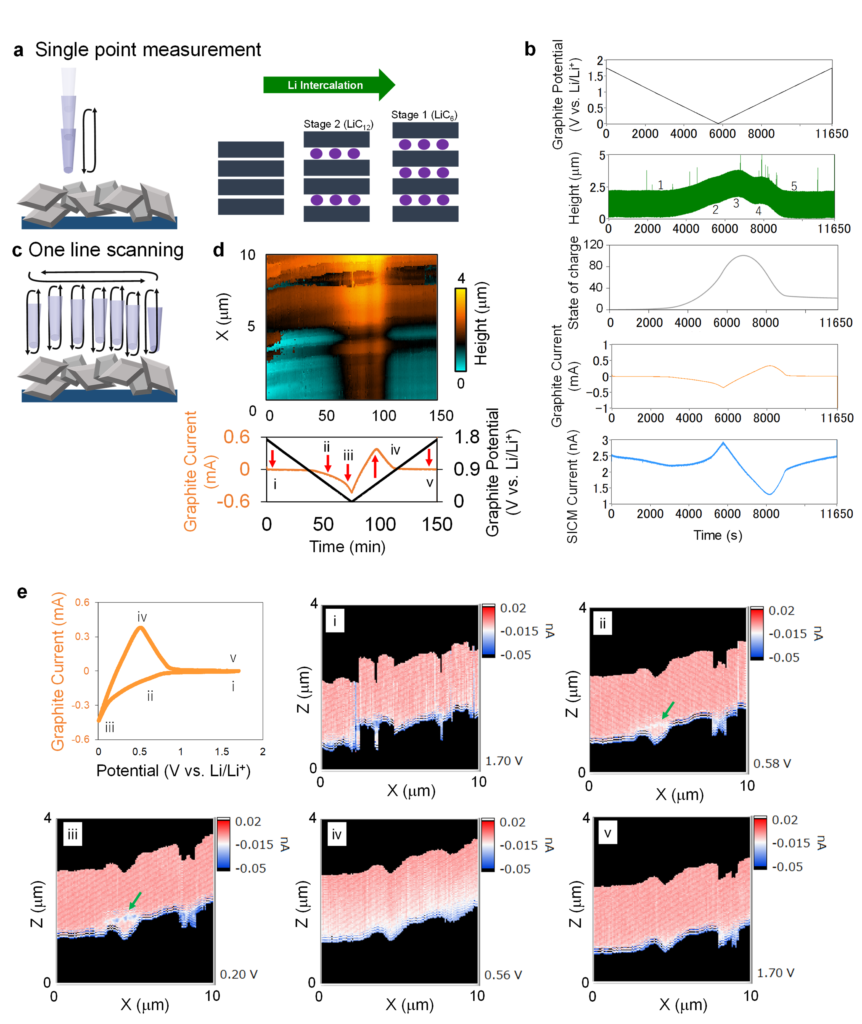
Lithium ion batteries dominate the energy storage sector from the scale of small portable devices to electric vehicles and even grid-scale electricity suppliers. Research is constantly ongoing to improve their energy density, charging speed, lifetime and safety, among other attributes. Here an understanding of not just the changes that go on in lithium ion batteries but how different parameters might interact can be extremely advantageous. Now researchers led by Yasufumi Takahashi and Takeshi Fukuma at Kanazawa University in Japan report simultaneous measurements of topography and ion concentration profiles by developing their operando scanning ion conductance microscopy (operando SICM). The combined data they retrieve can enable evaluation of correlations between the two parameters for improving future battery performance.
As Takahashi and Fukuma list in their report on the work, several processes are involved in the charging and discharging of lithium batteries, including the transport, solvation or desolvation, and intercalation of lithium ions, as well as structural changes and expansion in the electrodes, and the formation and deposition of by-products. These all occur out of equilibrium under applied electric potentials. “Capturing such multi-step and time-dependent changes with a relevant spatiotemporal resolution enables optimizing the operating conditions,” they point out highlighting design features that might benefit such as the structure of electrodes and separator, and tailoring additives to ensure proper solid−electrolyte interphase formation. As a result, numerous techniques have been used to investigate how both the surface topography and the ion concentration change of the anode/cathode during charging/discharging, all with different limitations.
A key advantage of SICM is that it can measure surface morphology and properties, including changes in ion concentration with depth in the electrolyte. Until now, however, no-one had retrieved simultaneous topographical and ion concentration data of a battery during charging and discharging.
The SICM uses a nanopipette containing an ionic solution as the scanning probe. The nanopipette acts as a probe and monitors changes in ion current from which it is possible to visualise ion concentrations as well as the distance to a surface. To take both measurements simultaneously, the researchers made a number of adjustments to the hardware and software so that they could independently control the battery and scanning probe electrode potentials. They also had to deal with various complicating factors such as the decrease in effective potential due to uncompensated solution resistance and the different settings of the potentiostat and amplifier used in the adjustments.
With these modifications, they were able to perform simultaneous topography and ion concentration profile measurements with a spatial resolution that exceeds optical microscopy. They were able to identify reversible phase changes during charging and discharging, as well as structural changes that were not restored on discharging the anode – a potential source of degradation. The researchers conclude that their “Operando SICM has the potential to unveil the lithium ion battery’s non-equilibrium mechanism and bottleneck processes such as dendrite formation by correlative analysis of the dendrite formation and ion concentration for optimizing the separator structure.”
Background
Scanning ion conductance microscopy (SICM)
The SICM is a scanning probe technique where a nanopipette uses as the probe. It monitors ionic current. As the gap between nanopipette and surface decreases, there is less space for ion flow, inhibiting the current and thus allowing users to control nanopipette-sample distance, as well as to get a measure of the topography. A hopping mode, where measurements are taken as the nanopipette is lowered and retracted can be used to avoid contact with the sample. The ion current also gives a measure of the ion concentration. However, while both modalities are possible, until now no-one has successfully retrieved both types of data simultaneously.
Yasufumi Takahashi and Takeshi Fukuma made a series of modifications to their SICM that allows them to collect ion concentration profile and topography information at the same time. They have successfully demonstrated their “operando SICM” on a lithium ion battery during charging an discharging, highlighting its potential to evaluate correlations between topographic and ion concentration changes to engineer better batteries.
Funder
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (19H00915, 15H05422, 15K13263, 20K21141, 16H00885), World Premier International Research Center Initiative (WPI), MEXT, Japan, Asahi Glass Foundation, Grant-in-Aid for Young Scientists provided by Hokuriku Bank, Murata Science Foundation, and Toyota Mobility Foundation (TMF).
Figure. Volume change monitoring during the CV of the graphite anode by Operando SICM.
(a) Scheme of the single point measurement. (b) Time course of the potential, height, state of charge, carbon anode current, and SICM current during the CV. (c) Scheme of the continuous one-line scanning measurement. (d) Time course of the one-line height image, carbon anode current, and SICM current during the CV. The sweep rate was 0.3 mV/s. (e) SICM ion current XZ images, where the image points are shown in Fig. 5d. Hopping amplitude and the falling rate of SICM were 2000 nm and 30 nm/ms, respectively. The nanopipettes radii were 50 nm and filled with 1.0 M LiClO4 in EC / DEC = 1 / 2 (v/v). The height monitoring was acquired by applying the constant voltage of 0.7 V vs. Li/Li+.



 PAGE TOP
PAGE TOP