Researchers at Kanazawa University report in Nature Communications how they can control chirality inversion in α helical peptides.
————————————————————————————————————————————————————————————————
The function of a protein is determined by its structure – prompting great interest in how to manipulate these structures. The structure is defined not just by the sequence of amino acids that make it, but the shape these acids make – the secondary structure – as well as how that shape is then folded. The most common secondary protein structure is the α-helix, which can coil to the right (P) or left (M). This coiling direction in turn determines how it engages with other chiral structures, which may be the form of a light beam or another molecule. Although molecular components and environmental factors can favor a particular coiling direction over the other, helical molecules tend to flip between the two coil directions. Now Naoki Ousaka, Mark J. MacLachlan and Shigehisa Akine at Kanazawa University in Japan have shown how they can control and fix the coil direction.
Helical proteins are chiral molecules, which means that the molecule’s shape cannot be fitted into its mirror image. In nature helical proteins often have other chiral components, such as sugars or amino acids, and these will determine which way the protein coils. However, there is a lot of interest in synthesizing artificial helical proteins that have different chemical components and hence functions not found in nature, and these may not have other chiral components. Nonetheless having both types or “enantiomers” of the chiral molecule can be hazardous because of the significant differences in behavior between the two chiral forms, one of which may be benign or even therapeutic while the other is toxic. Hence, there is demand for other ways of selecting and fixing the chirality.
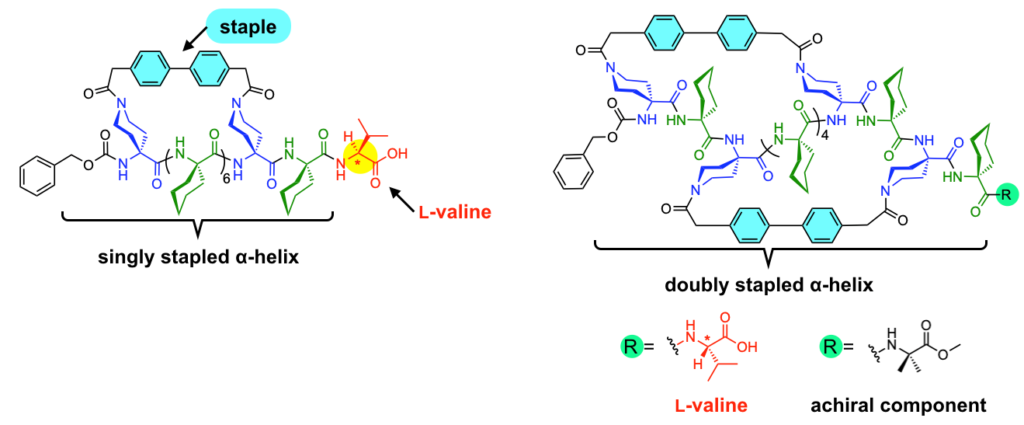
Ousaka, MacLachlan and Akine synthesized α helical molecules solely from achiral components. They included bulky segments so that the molecule tended towards the larger rings of the α helical structure, as well as side chains of piperidine – molecular components that are common in pharmaceuticals. These side chains can be cross linked to “staple” the molecule into either the righthanded or lefthanded coil, inhibiting flipping between the two – chiral inversion. Finally they added another molecular component, known as an ester – the L-Val-OH residue. This would switch the direction of the coil in response to acidic or basic environments due to preferences in the interaction between oxygen atoms in the ester and the amino acid backbone (Fig 1).
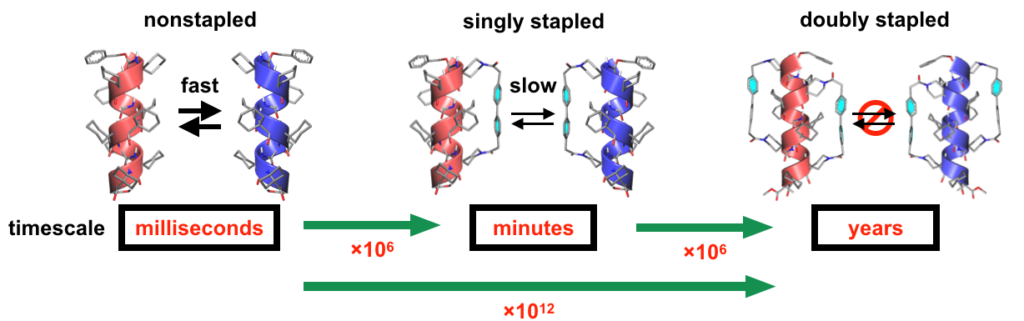
The researchers used a range of chiral characterization methods including circular dichroism, nuclear magnetic resonance and liquid chromatography. They found that with the molecule stapled just once, it would slow down the flipping between enantiomers by a factor of 106, although this still occurred over minutes. Changing the solution from to acid or alkali also successfully determined which enantiomer was favoured. However, stapling the molecule twice slowed down the chirality inversion by a factor of 1012, so that the molecular chirality was stable for years (Fig 2). This increased energy barrier to chirality inversion could then be overcome by heating the sample to very high temperatures to switch between the enantiomers.
Fig. 1: Molecular structures of singly and doubly stapled α-helix. The rigid biphenyl structure was used as the staple. The L-valine group is introduced at the terminal in order to determine the timescale of P/M inversion by acid/based addition. © 2023 Ousaka, et al., Nature Communications
Fig. 2: Timescales of P/M inversion of the nonstapled, singly-stapled, and doubly stapled α helices. Each stapling increased the timescale by approximately 106 times so that the double stapling effectively prevented from the racemization of the α-helix. © 2023 Ousaka, et al., Nature Communications
Glossary
The α-helix
Protein function is determined by its structure at a number of levels. The primary structure is given by the ordering of the amino acids that forms the polypeptide backbone of the protein. The polypeptide then forms a secondary structure, which may be “β sheets” or “α helices”, as well as a few other less common secondary structures. The α-helix is the most common secondary structure and forms a coil. Finally, these secondary structures fold into a conformation that gives the protein its the tertiary structure.
Hydrogen bonds crossing the helical turns that bond between specific atoms in each peptide describe additional rings in the structure. The α-helix is defined by having 3.6 amino acids in each turn such that each amino acid residue wraps itself around 100°, and 13 atoms in each ring.
Chiral molecules
Chiral molecules come in two types or enantiomers that are mirror inversions of each other and cannot be superposed. The type is often referred to as the handedness of the molecule, likening them to the right and left hand, which also cannot be superposed.
Chirality often results from a “chiral centre” such as a carbon atom, where the four limbs describing its tetrahedral bonding directions offer the opportunity for two arrangements of the groups on each limb that are mirror images of each other. This type of chirality is traditionally considered fixed, although researchers have recently demonstrated that it is possible for one enantiomer to convert to the other for these molecules (www.doi.org/10.1038/s41557-023-01156-7)
Another source of chirality is molecules with some kind of helical structure that can either spiral one way or the other. Helical chirality is known to be able to switch between one enantiomer and the other but until now it has not been possible to both accelerate and decelerate the process.
Circular dichroism
One of the oldest and still most widely used methods for determining chirality is measuring the “circular dichroism” of the sample. This uses illumination with a circularly polarized light beam where the direction of the electric field describes α-helix as the beam moves through space. The absorption of this circularly polarized light differs depending in whether the “handedness” of the light beam coincides or is opposite to that of the chiral molecule.
NMR
Nuclear magnetic resonance looks at the response of nuclear spins to magnetic fields. It gives an indication of the environment of the nucleus and so the molecular structure. The researchers also defined the binding constants of the molecules by integrating the NMR spectra obtained.
Liquid chromatography
This separates molecular components of a liquid depending on their size and/or structure. The liquid is drawn up some sort of stationary phase – traditionally paper or cloth but now often a gel – for example, by capillary action such that the larger molecules take longer than the smaller molecules. By incorporating chirality in a derivatizing agent, or the mobile or stationary phase, the different enantiomers of a sample can be separated.
Reference
Authors: Naoki Ousaka Mark J. MacLachlan and Shigehisa Akine.
Title: Stapling strategy for slowing helicity interconversion of α-helical peptides and isolating chiral auxiliary-free one-handed forms
Journal: Nature Communications
Published on Oct. 26, 2023.
DOI: 10.1038/s41467-023-42493-y
URL: https://doi.org/10.1038/s41467-023-42493-y



 PAGE TOP
PAGE TOP