Abstract:
The coordinated mechanisms balancing promotion and suppression of dendritic morphogenesis are crucial for the development of the cerebral cortex, the center of higher brain functions. Herein, the transcription factor Sox11 was identified as a suppresser of the dendritic morphogenesis and, therefore, the cerebral cortex development. Since Sox11 has been reported to be involved in the autism and intellectual disability, the current finding is expected to lead to better understanding of mental illnesses and developmental disorders.
The cerebral cortex is the center of higher brain function, attracting special attention in the research field of neuroscience. It is also considered to be the site related to various mental and neurological disorders. Abnormality of the cerebral cortex during development is thought to be one of the most serious causes of various neurological disorders. It is, therefore, essential to elucidate the mechanism underlying sound development of the brain, but there remain a number of problems unsolved. So it is a very important research domain of the brain science and medicine.
A neuron exchanges information with other neurons via a special cellular structure called the dendrite and the axon. At early stages of development, just after the birth, dendritic morphogenesis (dendrite development) begins inside of baby brain. If dendritic morphogenesis does not take place in an ordinary manner during this period of development, abnormalities arise, leading to severe disorders of the brain.
The present research team has looked for genes that play important roles at early stages of development of the cerebral cortex, and has found that the transcription factor Sox11 suppresses the dendrite development and that the loss of Sox11 triggers the dendrite development. This means that Sox11 is one of the crucial genes inhibiting the dendritic morphogenesis. There have been few reports about inhibitory genes for the dendritic morphogenesis. The coordinated mechanisms balancing promotion and suppression of dendritic morphogenesis are crucial for the development of the cerebral cortex. So far, genes to promote the dendritic morphogenesis have been known. On the other hand, the current study has identified a transcription factor that suppresses the dendritic morphogenesis for the first time. Sox11, a member of Sox family transcription factors, is known to be important for the body formation at fetal stages as other Sox family members. Since abnormality of Sox11 has been reported by other research groups in patients with autism-related diseases and with intellectual disability, the current finding is expected to lead to better understanding of those diseases and to development of their medical treatments.
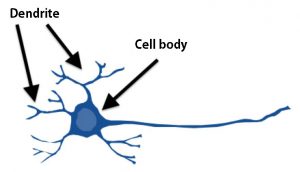
Figure1: Structure of a neuron in the cerebral cortex
Dendrites extend from the cell body, functioning for receiving information from other neurons.In various brain diseases, abnormalities of dendrites are observed, which may cause dysfunction of information exchange between neurons.This is considered to be one of the reasons for the phenotypes of these diseases.
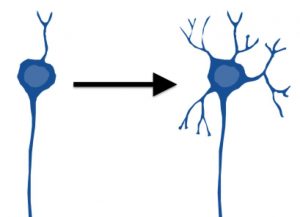
Figure2: Development of dendrites in the brain of babies.
At early developmental stages, i.e., just after birth, dendrites are not yet well formed, but along with body growth, dendrites develop.The sound development of dendrites is believed to be important for acquiring normal functions of the brain.
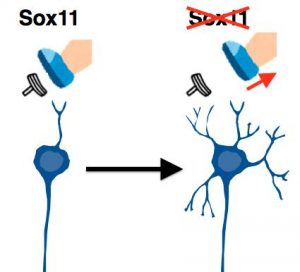
Figure3: Sox11, a crucial gene to suppress dendrite development
This study has demonstrated that Sox11 is a crucial gene to suppress dendrite development.Loss of Sox11 gene was found to trigger dendrite development.
Article
Title: Sox 11 Balances Dendritic Morphogenesis with Neuronal Migration in the Developing Cerebral Cortex
Journal: The Journal of Neuroscience
Authors: Yoshio HOSHIBA1,2, Tomohisa TODA1,2, Haruka EBISU1,2, Mayu WAKIMOTO1,2, Shigeru YANAGI3, Hiroshi KAWASAKI1
1Kanazawa University, 2University of Tokyo, 3Tokyo University of Pharmacy and Life Sciences
Doi: 10.1523/JNEUROSCI.3250-15.2016
Funder
Grants-in-aid from MEXT and from JSPS



 PAGE TOP
PAGE TOP