Abstract:
An international team led by a researcher in Kanazawa University had found a previously unknown relationship among the growth factor signaling, multipotent stem cells, and a unique growth spurt so-called “catch-up growth”, by the study using fish embryo model. Since the catch-up growth often associates with adult-onset disorders, this study may open up new avenues for further understanding of yet obscure molecular and cellular mechanisms of the catch-up phenomenon and the related pathogenesis.
[Background]
Animal growth is cooperatively defined by genetic and epigenetic factors. In a rapidly growing human fetus, an adverse environment such as lower oxygen and malnutrition by dysfunctions of placenta and umbilical cord often lead to developmental arrest or intrauterine growth restriction (IUGR) *1). Intriguingly, once an unfavorable condition is removed at the birth, most of stunted IUGR-infants restart growth with an accelerated progression rate, which is referred to as “catch-up growth” (Figure 1). Cumulative epidemiological data strongly suggest that IUGR and subsequent catch-up growth associates with adult-onset disorders such as obesity, type-II diabetes, and cardiovascular diseases. Since the changes during such an anomalistic growth pattern seem to be a key for deciphering the cause of future pathogenesis, we have been looking for a detailed molecular and cellular basis of the catch-up phenomenon. Studies using rodent models such as mouse and rat, however, originally have technical complexities and difficulties for observing and controlling the growth of intrauterine specimens, which had made it very difficult to do the related research.
A research group at Kanazawa University, Japan, has been using the zebrafish (Danio rerio) *2), a model species preferentially used for the animal growth and development, to tackle this issue. The zebrafish embryo grows constantly under normal oxygen condition (normoxia) but the growth rate is easily blunted under low oxygen environment (hypoxia). By using the zebrafish embryo, the research group previously developed an experimental model of hypoxia-induced growth retardation and the subsequent reoxygenation-induced catch-up growth; they also reported that insulin/insulin-like growth factor signaling (IIS) *3) was uniquely modified by the change of environmental oxygen level to induce the catch-up growth under reoxygenation. This time, in the course of the study, they have identified a previously unknown participation of a multipotent stem cells in the catch-up growth.
[Results]
The research group focused on a gene named insulin receptor substrate (irs)1, encoding an intracellular signaling mediator protein that initiates and sustains the IIS in target cells. They first characterized irs1 gene in zebrafish and found that the reduced expression of irs1 gene by the loss-of-function approach attenuated early embryonic IIS and severely hampered catch-up growth (Figure 2a). Inhibition of irs1 gene expression led to noticeable cell death in neural crest cells (NCCs) *4), a multipotent stem cells emerged at early embryogenesis, under hypoxia (Figure 2b). Furthermore, they impaired the NCC development without changing irs1 expression by pharmacological and genetic approaches and found it significantly hindered the catch-up growth (Figure 2c). Meanwhile, prevention of the apoptotic pathway by pan-Caspase inhibition or by forced activation of IIS in irs1 knocked down embryos blocked cell death in NCCs, and the catch-up growth was rescued. These data indicate an indispensable role of irs1 for NCC survival under hypoxia, which is a prerequisite for the hypoxia/reoxygenation induced catch-up growth (Figure 3).
[Significance and future prospects]
The current study first unveiled the importance of the multipotent stem cells for the catch-up growth phenomenon. In the light of the fact that NCCs harbor great potentials to be various types of cells, it is likely that the behaviors and cell-fates of the stem cells (or its derivatives) are uniquely modified during IUGR and catch-up growth. Obesity, cardiovascular diseases, insulin resistance, delayed mental development, and other unfavorable physiological outcomes are often found in individuals who experienced severe growth delay and catch-up growth in early stages of life. Currently unknown molecular and cellular changes in NCCs during the IUGR/catch-up growth may bring an important clue for deciphering the underlying mechanisms of such pathogenesis. Thus, this study would provide a novel hypothesis and further opportunity for better understanding of the catch-up growth.
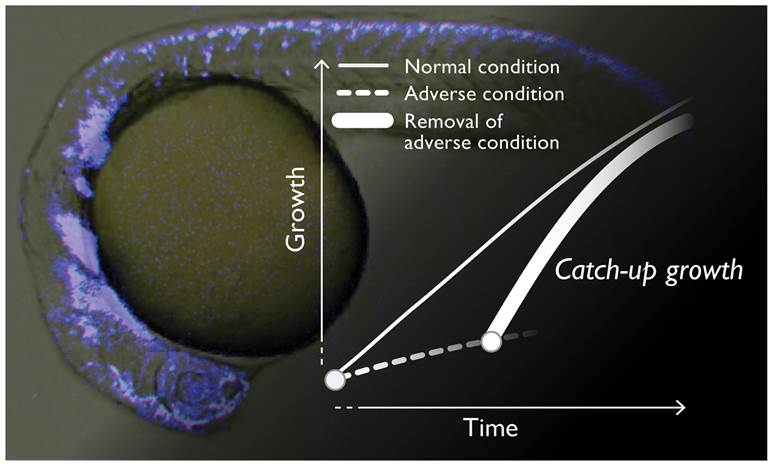
Figure 1.
A conceptual diagram of the catch-up growth. The neural crest cells (NCCs) in a zebrafish embryo are visualized by a fluorescent signal (Background picture).
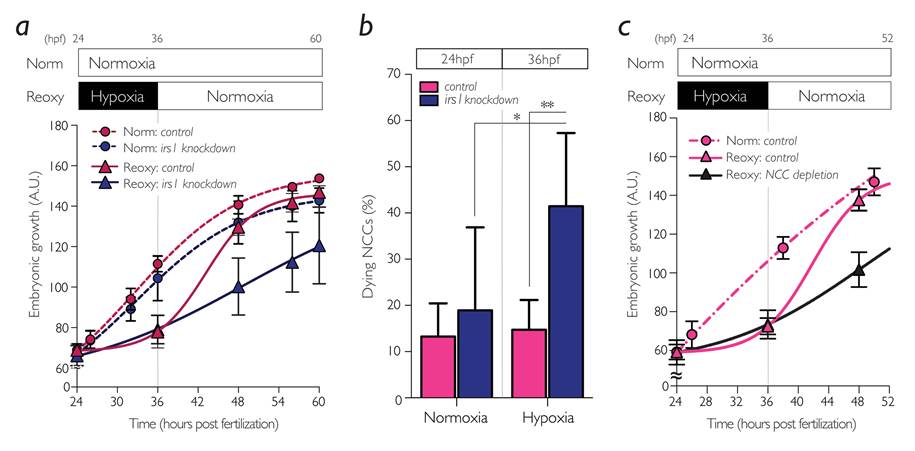
Figure 2.
Irs1 and NCCs were required for reoxygenation-induced catch-up growth in zebrafish embryo. (a) Growth curves and (b) NCC survival of the irs1-knocked down embryos. (c) Growth curves of the NCC-depleted embryos. All data are shown as means±S.D., *, P<0.05; **, P<0.01.
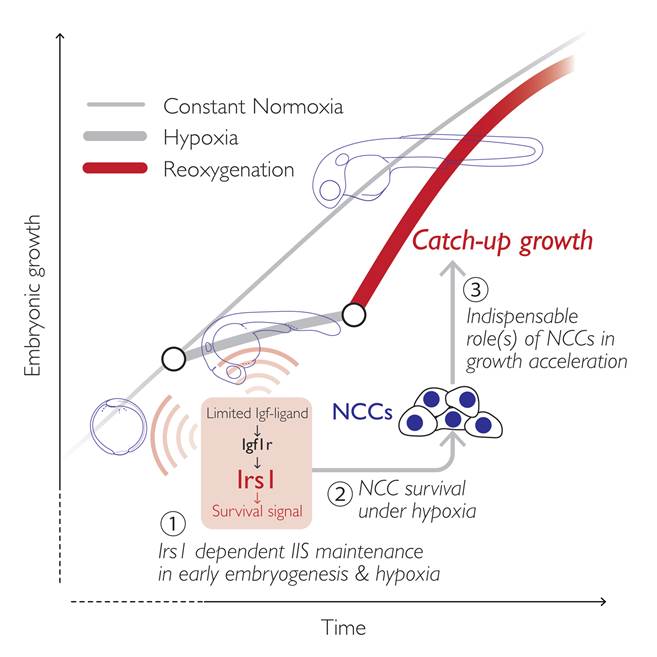
Figure 3.
A proposed model. The Irs1-mediated IIS in developing embryos is important for NCC survival under severe hypoxia (1, 2), and the NCCs are indispensable for reoxygenation-induced catch-up growth (3).
[Glossary]
*1) Intrauterine growth restriction
Poor growth of a fetus during pregnancy. Though there are many causes for this, poor nutrition and lack of oxygen supply to the fetus are often known to be involved.
*2) Zebrafish (Danio rerio)
A freshwater fish in Cyprinidae family. Their embryo is a widely-used vertebrate model in many biology research. Its fast and conserved development, availability of established experimental methods, extra-uterine development, and the ease of manipulating environmental conditions are all well-suited especially for developmental studies.
*3) Insulin/insulin-like growth factor signaling
Insulin/insulin-like growth factor (Igf) signaling (IIS) is a major hormonal pathway facilitating body growth in a wide range of metazoans. The insulin and Igf ligands bind to the insulin receptor or type 1 Igf-receptor (Igf1r), which triggers activation of the receptor tyrosine kinases and subsequent tyrosine phosphorylation of specific substrates, the insulin receptor substrates (Irs: Irs1-4). The tyrosine phosphorylation of Irs activates several major downstream pathways such as PI3K-Akt pathway and Ras-Raf-Mek-Erk1/2 pathway, and regulates numbers of cellular behaviors (i.e. cell survival, proliferation, differentiation, and migration).
*4) Neural crest cells
Multipotent stem cells that are emerged at the border of the neural plate and the non-neural ectoderm during early development. NCCs are to be various types of cells such as cartilages, cranial bones, connective tissues, muscles, glia, neurons, pigment cells.
Article
Catch-Up Growth in Zebrafish Embryo Requires Neural Crest Cells Sustained by Irs1 Signaling
Journal: Endocrinology
Authors: Hiroyasu Kamei, Yosuke Yoneyama, Fumihiko Hakuno, Rie Sawada, Toshiaki Shimizu, Cunming Duan, Shin-Ichiro Takahashi
Doi: 10.1210/en.2017-00847
Funders
Grants-in-Aid (A)(2) 16208028, (A) 22248030, (A) 25252047, and (S) 25221204l; Challenging Exploratory Research Grants 20658065, 25660210, and 15K14840l; the core- to-core program from the Japan Society for the Promotion of Science (JSPS) and Program for Promotion of Basic and Applied Researches for In- novations in Bio-oriented Industry (25006A) (to S.-I.T.), and Grant-in-Aid (B) 243801521 (to F.H.). This work was also par- tially supported by Grants-in-Aid for Young Scientists (Start-up) 23880008, (B) 25850220, and (B) 15K18799 (to H.K.) from JSPS. H.K. was supported by National Institute of Genetics, Cooperative Research Program Grants 2012-B11, 2013-A28, and 2014-A26.



 PAGE TOP
PAGE TOP