Abstract:
GroEL, an ATP-fueled protein machine with a double-ring structure, assists proper folding of many proteins, in cooperation with its co-chaperonin, GroES. The team of Prof. Toshio Ando and Dr. Daisuke Noshiro in NanoLSI directly observed dynamic interactions between GroEL and GroES during the chaperonin reaction, using high-speed atomic force microscopy (HS-AFM). They succeeded for the first time in elucidating the entire reaction scheme of this complicated system and specifying inter-ring allosteric communications that grant high efficiency to the chaperonin reaction.
[Background]
GroEL is a cylindrical protein complex formed by two heptameric rings stacked back to back, each consisting of identical ATPase subunits. GroES is a single homo-heptameric ring and binds to the ends of the GroEL cylinder depending on the nucleotide state of GroEL. Each ring of GroEL has a cavity, in which denatured protein substrate can be encapsulated and eventually renatured. GroES functions as a lid of the cavity. Since this chaperonin system contains multiple components, i.e., 14 ATPase sites, two rings of GroEL, protein substrate, and co-chaperonin GroES, it has been difficult to decipher how the chaperonin reaction proceeds. In particular, the most interesting issue of how two rings communicate with each other to achieve high efficiency for the reaction has long been elusive.
[Results]
By using HS-AFM, this team directly observed dynamic binding and release of GroEL at the two rings of GroEL during the ATPase cycle (Fig. 1), and thereby precisely measured the interaction patterns and the rate of each reaction step. The observation was performed under different substrate conditions: in the absence of substrate protein and in the presence of different types of substrate proteins. Since the results were not largely dependent on the substrate condition, descriptions of the substrate dependence are omitted here. The reaction intermediates observed were mostly GroEL : GroES2 complexes and GroEL : GroES1 complexes. From their shapes, the former is called football complexes (abbreviated as F) and the latter bullet complexes (abbreviated as B). The GroES-bound ring is called as the cis-ring, while the GroES-unbound ring is called as the trans-ring. The trans-ring is formed when the ring contains only ADP produced by ATP hydrolysis into ADP−Pi and then Pi release. Therefore, in football complexes the two rings are either in the ATP or ADP−Pi bound state. Each ring consists of seven subunits. From biochemical analyses, bound seven ATP molecules are considered not to be hydrolyzed independently but hydrolyzed all together. The HS-AFM imaging revealed that the reaction patterns are largely classified into two types: main and side pathways. In the main pathway, GroES binding and release occur alternately between the two rings, as
B↑ → F → B↓ → F,
where the vertical arrows indicate the polarity of bullet complexes (the GroES-bound ring appears sharper than GroES-unbound ring). In the side pathway, this rhythmic, alternate reaction is disrupted and no polarity switch occurs, as
B↑ → F → B↑ → F
(see images at 5.52 s, 5.98 s and 6.21 s in Fig. 1b). Here, we describe mostly for the main pathway. In this pathway, alternate binding and release of GroES occur between the two rings. Hence the two rings must mutually sense the counter ring’s nucleotide states or GroES-bound/GroES-unboud states. That is, there must be inter-ring allosteric communications.
From the lifetimes of bullet and football complexes, the B-to-F and F-to-B transition rates, kBF and kFB, were determined. Since the HS-AFM observations were performed in the presence of high-concentrations of ATP and GroES, ATP binding and the following GroES binding occur almost instantaneously. Therefore, kBF represents the rate of ADP dissociation from the trans-ring in the bullet complexes. kFB represents the rate of GroES dissociation from the first (older) cis-ring in football complexes, and hence the rate of Pi release from the ADP−Pi bound to the cis-ring. Moreover, the residence time distribution of bound GroES (i.e., the time period that the bound GroES spends until its final dissociation) was best fitted to a sequential four-step reaction model described with four rate constants (k1, k2, k3 and k4), including the rate constant for the final GroES release step. Therefore, the bound GroES undergo four intermediate states. The intermediates that AFM has detected during this period are only three: the first football, the bullet following the football and the second football. Therefore, there is an additional intermediate that cannot be distinguished from its topography. Although explanations are omitted here, it was clarified that a different football (F*) is formed just after the first football (Fig. 2). The values of four rate constants for transitions from one state to the next, including the rate for the final dissociation of GroES, were determined from the above mentioned fitting. Remarkably, the following coincidences appeared (see Fig. 2):
(1) k4 = kFB,
(2) k3 = kBF, and
(3) 1/k1 + 1/k2 = 1/kFB.
The first coincidence is trivial, since both k4 and kFB represent the same rate, i.e., the rate of GroES dissociation from the same ring (the top ring in Fig.2). Unlike this, k3 represents a rate of state transition occurring in the top ring, whereas kBF represents the rate of GroES binding to the opposite ring. Therefore, the second coincidence indicates the existence of inter-ring communications that lead to synchrony between transitions occurring at the two rings. So does the third coincidence.
Since the second (older) cis-ring in the final football transits into the ADP-bound trans-ring in the final bullet (see Fig. 2), the second cis-ring must contain ADP−Pi. Therefore, k3 represents the rate of ATP hydrolysis (ATP→ADP-Pi) on the top ring (Fig. 2). kBF represents the rate of ADP dissociation from the trans-ring (the bottom ring of the second bullet in Fig. 2). Hence, the coincidence k3 = kBF indicates that ATP hydrolysis to ADP−Pi triggers ADP release from the opposite ring, and vice versa. This inter-ring regulation is functionally important. Without this regulation, ADP dissociates quickly immediately after the formation of bullet, which is immediately followed by ATP binding and then GroES binding. As such, the encapsulated substrate protein would lose a chance to be released from the trans-ring. We can interpret the second coincidence to mean that ATP hydrolysis to ADP−Pi in the cis-ring acts as a timer to determine the time when ADP is released from the opposite ring. It is difficult to interpret the third coincidence, because we do not yet know the state of F*. However, it is sure that a certain change occurring after ATP binding (possibly a structural change enabling the substrate protein encapsulation into the cavity) is coupled with Pi release from the opposite ring. Thus, it was elucidated that the two events, ATP hydrolysis to ADP−Pi and Pi release, act as timers, according to which events in the corresponding opposite rings proceed.
Finally, the entire reaction scheme of the chaperonin reaction is shown in Fig. 3. This scheme was deciphered from HS-AFM observations in the presence of denatured maltose-binding protein (MBP). Minor pathways are also included in this scheme, in addition to the main and side pathways. The chaperonin reaction proceeds in a more complex and stochastic way than ever thought before. Such a complicated reaction scheme cannot be revealed with conventional approaches. The direct HS-AFM observation of dynamic events occurring at individual molecules and individual rings of GroEL made possible this detailed assessment of the highly complicated reaction. This study also presented an important issue to be tackled in the future: i.e., a mechanism by which the reaction branches into the side pathway. The side pathway occurs at a relatively high probability (1/3−1/4), and hence, a certain mechanism of the pathway branching must exist.
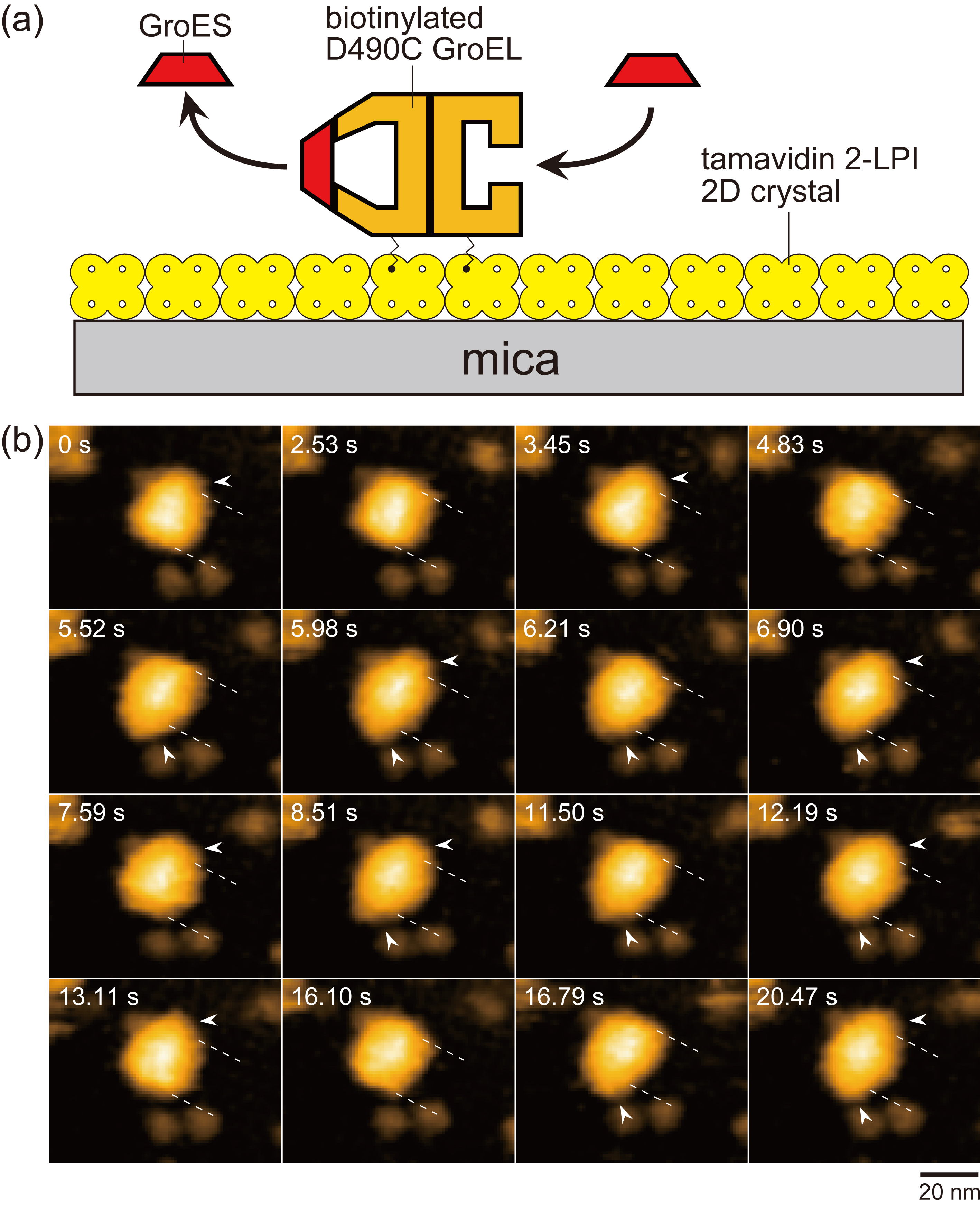
Figure 1.
Assay system for HS-AFM imaging of dynamic GroEL–GroES interaction and captured images. (a) Schematic of assay system used for HS-AFM imaging. Tamavidin 2-LPI was two-dimensionally crystallized directly on a bare mica surface. GroEL D490C biotinylated at Cys490 located at its equatorial domain was tethered to the tamavidin 2-LPI 2D crystal surface through the biotin–tamavidin 2-LPI linkage with a linker length of approximately 2.5 nm. Because this crystal surface is resistant to non-specific protein binding, the tethered GroEL does not adsorb onto the surface. (b) HS-AFM images showing repeated GroES association and dissociation at the two rings of GroEL in the presence of denatured MBP. The images are clipped from successive images captured at 4.35 frames/s. The dashed lines indicate the positions of toroid ends of the GroEL molecule. The arrowheads indicate GroES bound to GroEL. Besides football and bullet complexes, GroES–unbound GroEL also appeared at 2.53, 4.83 and 16.10 s. The bulk solution contains 1 μM GroES, 2 mM ATP and 200 nM denatured MBP.
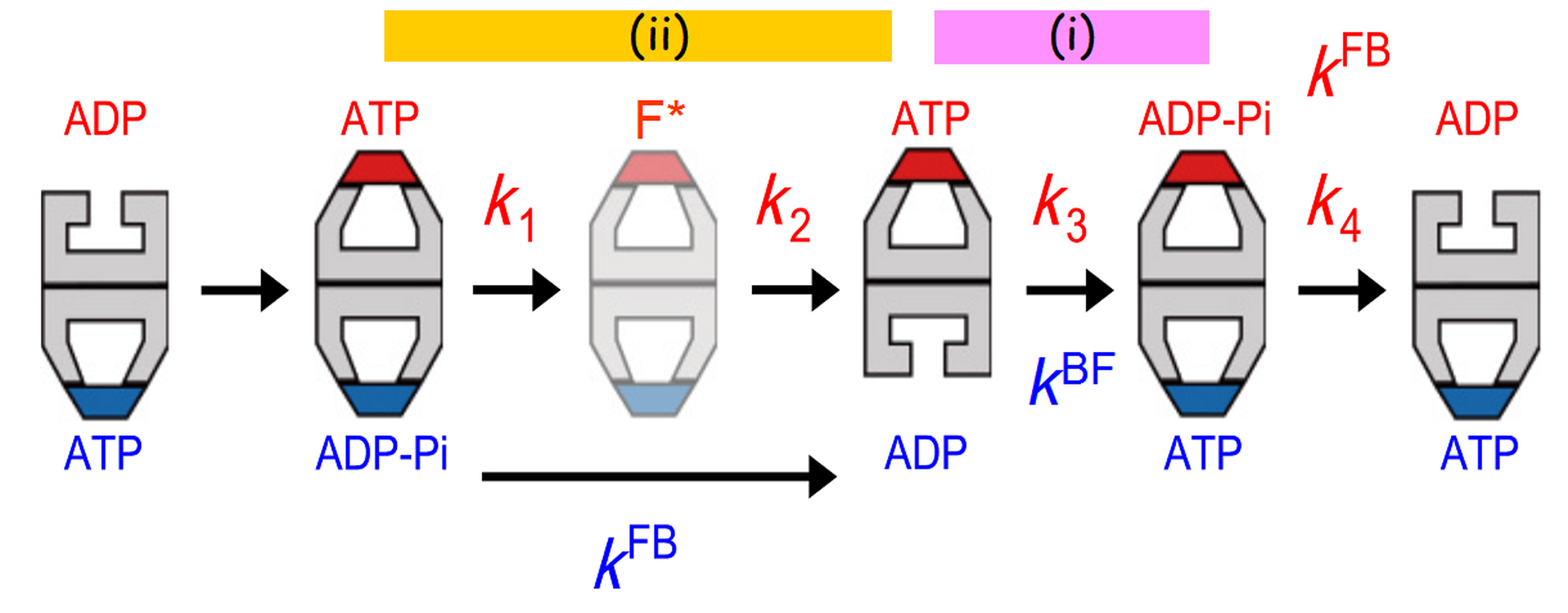
Figure 2.
Allosteric communications between two rings of GroEL. The football complex (F*) shown in pale colours are apparently the same as but kinetically different from the football complex formed immediately before. The residence time distribution of bound GroES was best fitted to a sequential four step reaction model with four rate constants, k1, k2, k3 and k4. These rate constants correspond to state transitions occurring at the top ring. The rate constants, kFB and kBF, shown in blue correspond to the F-to-B and B-to-F transitions occurring at the bottom ring. The transition steps that occur synchronously between the two rings are shown with the bars marked with (i) and (ii).
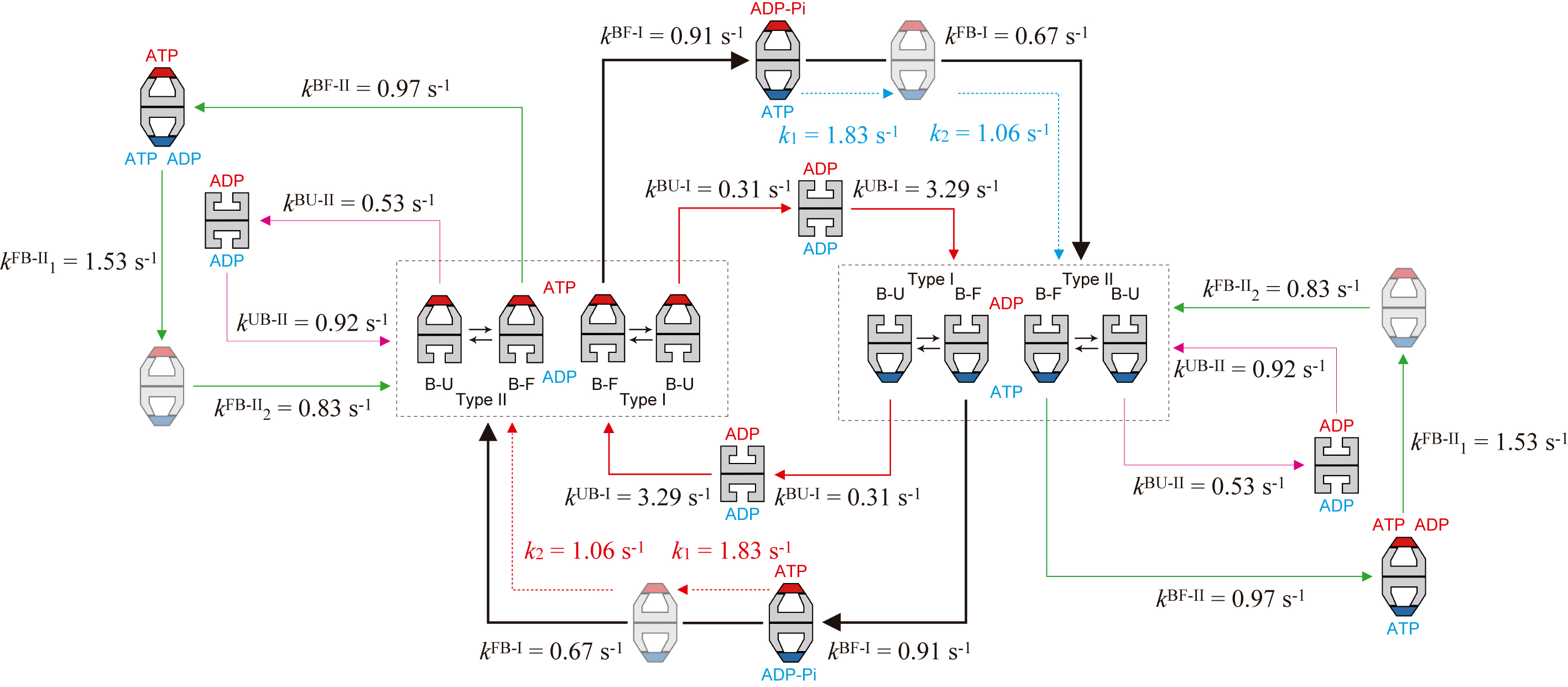
Figure 3.
Kinetic reaction scheme of GroEL–GroES interaction revealed by HS-AFM imaging in the presence of MBP. The thickness of arrows (shown with solid black, red, and green lines) along reaction pathways indicates the relative frequency of occurrence. The dashed red arrows indicate reaction processes occurring in the ring bound to GroES shown in red, while the dashed blue arrow indicates reaction processes occurring in the ring bound to GroES shown in blue. The order of k1FB-II and k2FB-II and the order of k1 and k2 are tentative. In the side pathway, the coexistence of ATP and ADP in one ring is shown but hypothetical. This hypothesis can possibly account for the mechanism of pathway branching into the side pathway, although it has to be proven in the future.
Article
Substrate protein dependence of GroEL–GroES interaction cycle revealed by high-speed atomic force microscopy imaging
Journal: Philosophical Transactions of the Royal Society B
Authors: Daisuke Noshiro, Toshio Ando
Doi: 10.1098/rstb.2017.0180.
Funders
This work was supported by grants to T.A.: KAKENHI, Grants-in-Aid for Scientific Research (S) (no. 24227005 and no. 17H06121); KAKENHI, Grants-in-Aid for Research on Innovative Area (research in a proposed research area; no. 26119003) and CREST programme of the Japan Science and Technology Agency (no. JPMJCR13M1). This work was also supported in part by KAKENHI, Young Scientists (B) (no. 17K15433 to D.N.).



 PAGE TOP
PAGE TOP