Abstract:
A research team based in Japan led by Kanazawa University has demonstrated the effect of osteopontin on hepatitis C virus replication and interferon signaling in cancer stem cells. Their research sheds light on a novel therapeutic target for treating hepatocellular carcinoma.
[Background]
Hepatitis C virus (HCV) infection is the major cause of hepatocellular carcinoma (HCC) and was estimated to be responsible for 745,000 deaths in 2012. Recently, highly efficient and direct-acting antiviral agents (DAAs) have been able to eliminate HCV from infected livers in more than 90% of cases. However, emergence of HCC at a rate of about 1% per year is now reported in HCV-infected livers. Therefore, new therapeutic strategies are needed to prevent HCV infection, HCC recurrence, and hepatocarcinogenesis.
Osteopontin (OPN) is a multifunctional cytokine and is involved in normal physiological processes, as well as in numerous pathological conditions, including inflammation, fibrogenesis, and carcinogenesis. In liver diseases, OPN plays an important role in acute liver injury, viral replication, liver repair, fibrosis, and HCC.
Recent work has identified CD44 as the most common marker for cancer stem cells (CSCs) in several human cancers. CD44 has a pivotal role in regulating the properties of CSCs, including their self-renewal, tumor initiation, metastasis, and chemoradioresistance, and OPN reportedly interacts with CD44.
In HCC, enrichment of several stem cell markers, including CD133, CD90, CD13, epithelial cell adhesion molecule (EpCAM), CD44, CD24, and oval cell marker OV6, is reported in certain side populations of CSCs. However, CSCs represent only a minor population of the cancer cells and there is currently no evidence for a role for CSCs in supporting HCV replication. Therefore, identifying the underlying mechanism of HCV pathogenesis and its relationship to CSCs is an important research challenge.
In this study, a group from Kanazawa University evaluated the significance of the OPN–CD44 axis for HCV replication in EpCAM+/CD44+ CSCs, and investigated the role of OPN in the regulation and maintenance of EpCAM+/CD44+ CSCs.
[Results]
EpCAM+/CD44+ CSCs showed marked HCV replication when compared with EpCAM−/CD44− cells. In addition, the levels of OPN mRNA and protein were higher
in EpCAM+/CD44+ CSCs than in EpCAM−/CD44− cells. OPN significantly enhanced HCV replication in EpCAM+/CD44+ CSCs and markedly suppressed interferon (IFN)-stimulated gene expression. Glycogen synthase kinase-3β inhibitor 6-bromoindirubin-3-oxime increased the EpCAM+/CD44+ CSC population and OPN expression and impaired IFN signaling via signal transducer and activator of transcription 1 (STAT1) degradation. Furthermore, OPN regulated stemness of EpCAM+/CD44+ CSCs, which led to inactivation of IFN signaling and enhanced HCV replication.
[Significance and future prospects]
The Kanazawa University group focused its attention on CSCs, as HCC is proposed to develop from CSCs, even though they represent a small part of the HCC cell population. However, HCV replication in CSCs is still poorly understood. This study showed the significance of the OPN–CD44 axis for HCV replication in EpCAM+/CD44+ CSCs.
The results of the Kanazawa University group highlight a new role for OPN in supporting HCV replication in EpCAM+/CD44+ CSCs through a reduction in STAT1 activation. They also provide evidence that OPN has the potential to maintain CSC phonotypes, and identify the OPN–CD44 pathway as a potential target for regulating HCV replication and stemness in HCC cells.
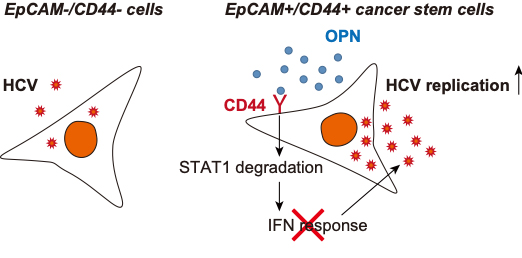
Figure 1. Schematic model of this study.
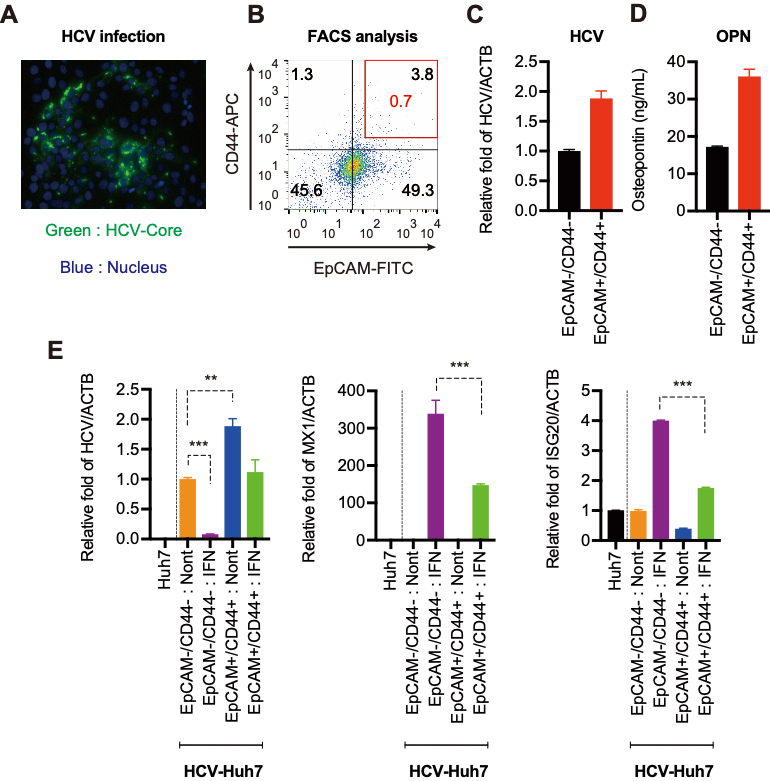
Figure 2. HCV replication is increased in EpCAM+/CD44+ CSCs.
(A) Immunofluorescence staining of HCV-core protein in HCV replicating Huh7 cells. (B) FACS analysis of EpCAM and CD44 expression in HCV replicating Huh7 cells. (C) RTD-PCR analysis of HCV-RNAin EpCAM+/CD44+ CSCs and EpCAM−/CD44− cells. (D) ELISA analysis of the level of OPN in EpCAM+/CD44+ CSCs and EpCAM−/CD44− cells. (E) RTD-PCR analysis of HCV-RNA, MX1, and ISG20 in Huh7, EpCAM+/CD44+ CSCs, and EpCAM−/CD44− cells following IFN treatment.
Article
The osteopontin-CD44 axis in hepatic cancer stem cells regulates IFN signaling and HCV replication
Journal: Scientific Reports
Authors: Takayoshi Shirasaki, Masao Honda, Taro Yamashita, Kouki Nio, Tetsuro Shimakami, Ryougo Shimizu, Saki Nakasyo, Kazuhisa Murai, Natsumi Shirasaki, Hikari Okada, Yoshio Sakai, Tokiharu Sato, Tetsuro Suzuki, Katsuji Yoshioka & Shuichi Kaneko
DOI: 10.1038/s41598-018-31421-6
Funders
This research was partially supported by AMED under Grant Number JP18fk0210046, JP18fk0210012, JP18fk0210020, JP17fk0210201, JP18fk0210005 and JP18fk0310110.



 PAGE TOP
PAGE TOP