Abstract:
In a recent study published in The Journal of Neuroscience, researchers reveal how the basic structure of the brain is formed.
The human brain consists of neurons arranged into microscopic columns. The cortex, which is the seat of most cerebral functions and forms the largest part of the brain, is divided into uncountable micro-columns. However, the exact development of this columnar structure is elusive to neuroscientists. A research team led by Makoto Sato, has recently reported their study describing the role of a specific protein in the growth of these columns.
The team spanning across Kanazawa University, Ryukoku University, Tokyo Institute of Technology and Imperial College London, used the Drosophila melanogaster (fruit fly) brain for their experiments. The visual centre of the Drosophila brain bears high structural resemblance to the columnar arrangement of the human one, making it an apposite, yet simple model to study. To first visualize these columns, N-cadherin (Ncad), a protein specific to the nervous system, was mapped. The spatial locations of Ncad revealed that during the larval stage of the fly, the visual centre comprised a donut-like structure. As the fly matured to the pupal stage, these structures started stacking on top of each other and indeed transformed into a three-dimensional column.
The research team then carefully analysed these columns and found the presence of three neuron types, namely, R7, R8 and Mi1 within them. While R7 was concentrated towards the central core of the columns, R8 and Mi1 were arranged towards the periphery. Now that the structural composition was clear, understanding the process of column formation was the next step. It was suspected that Ncad, which was heavily present in the columns, played a role in this regard. When Ncad was measured in all three neuron types, it was found that R7 neurons contained more Ncad than the peripheral neurons. Since Ncad is known to give cells adhesive properties, the team concluded that it was the levels of Ncad which determined the location of each of the neuron types within the columns. Heavily adhesive neurons such as R7 formed the core of the columns.
Lastly, to see whether Ncad directly affected the columnar arrangement, it was either completely removed or greatly increased in the neurons. As expected, manipulation of Ncad disturbed the columnar assembly and positions of the neurons. For example, R7 neurons without any Ncad were no longer within the core. However, when Ncad was greatly increased within them, the R8 and Mi1 neurons extended towards the core, possibly due to the strong adhesive properties of Ncad.
This study revealed the role of an adhesive protein in arranging neurons to form the columnar microstructure of the brain. “[Ncad-dependant] differential adhesion and inter-layer interaction may be the essential mechanism underlying the 3D organization of column formation that is evolutionarily conserved from the fly optic lobe to mammalian brains”, conclude the researchers. This discovery can help neuroscientists monitor healthy development of the brain and uncover other molecules involved in the process.
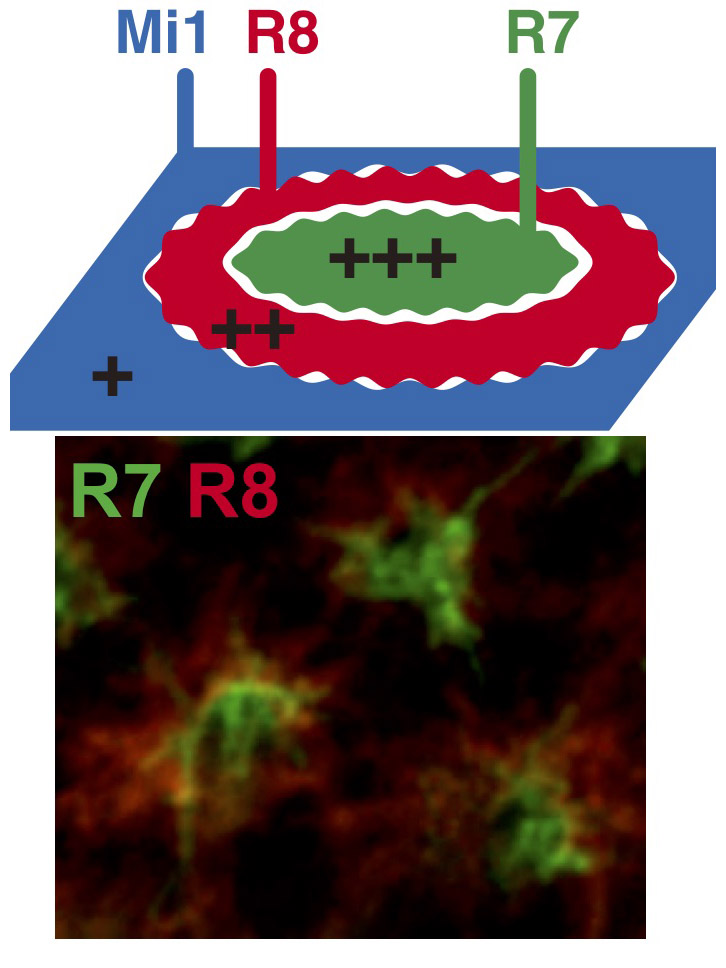
Figure 1.
R7, R8 and Mi1 form the basic structure of the column. The relative adhesiveness and Ncad expression level are in the order of R7 > R8 > Mi1 (top). Visualization of the terminals of R7 (green) and R8 (red) in the developing fly brain (bottom).
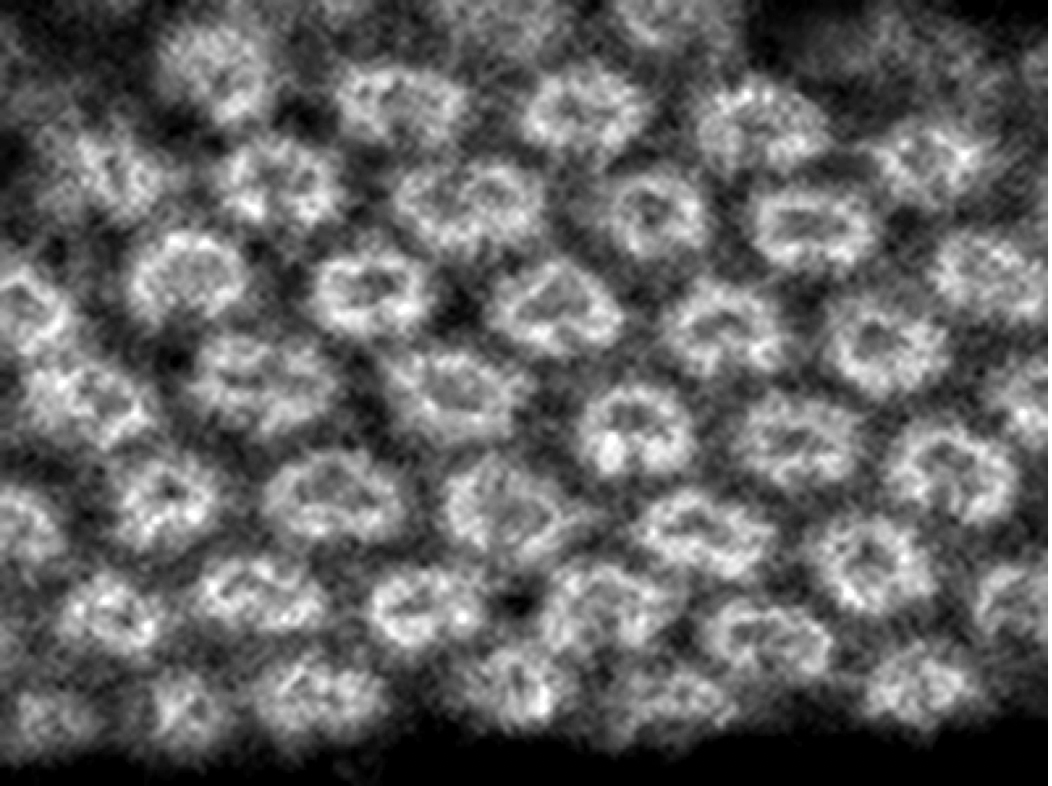
Figure 2.
Ncad distribution visualizes the regular arrangement of the donut-like columnar structures in the developing fly brain.
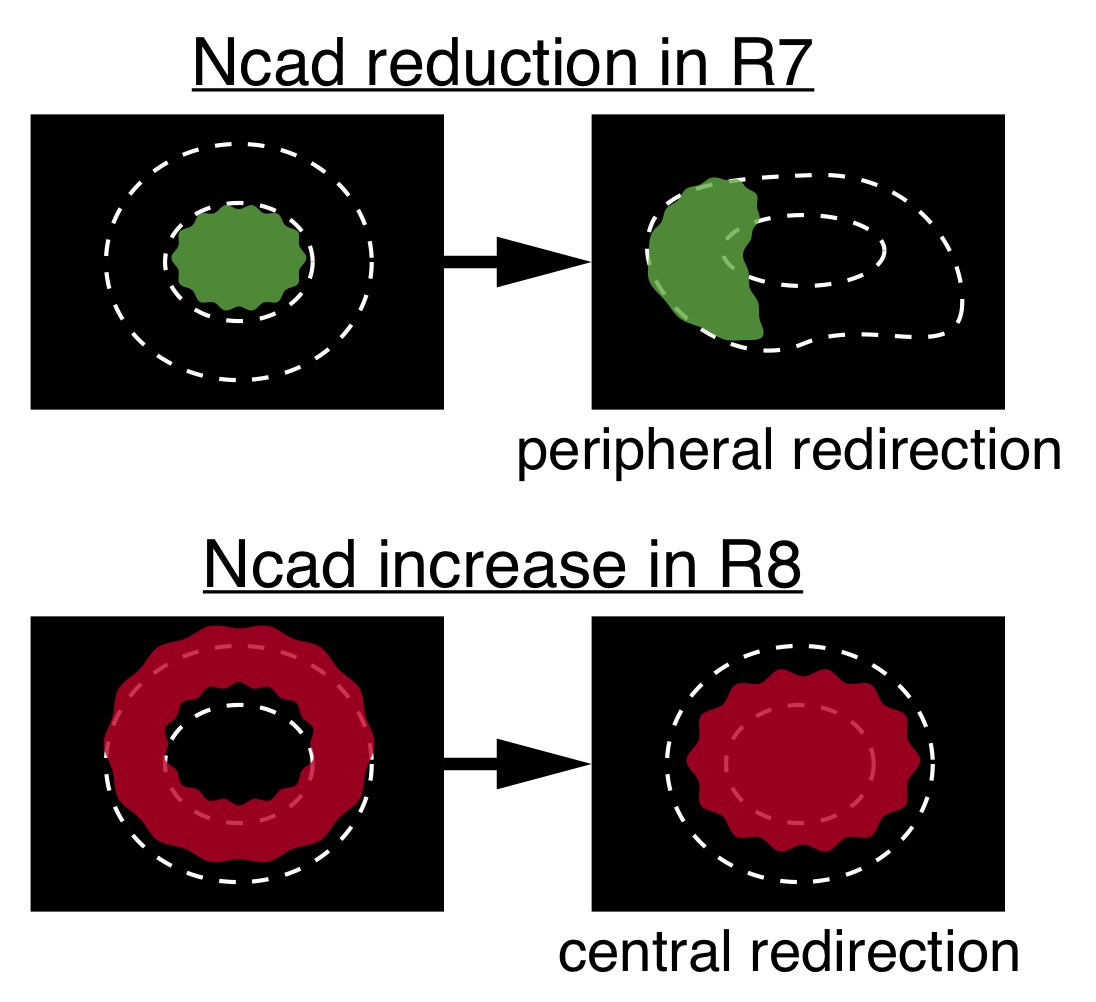
Figure 3.
When Ncad expression and adhesiveness were reduced in R7, which is usually situated at the central core region of the column, R7 terminals were redirected to the peripheral region of the column (top). In contrast, artificial increase of Ncad expression level and adhesiveness of R8 caused its redirection toward the central core region of the column (bottom).
Article
N-cadherin orchestrates self-organization of neurons within a columnar unit in the Drosophila medulla
Journal: The Journal of Neuroscience
Authors: Olena Trush, Chuyan Liu, Xujun Han, Yasuhiro Nakai, Rie Takayama, Hideki Murakawa, Jose A. Carrillo, Hiroki Takechi, Satoko Hakeda-Suzuki, Takashi Suzuki, and Makoto Sato
DOI: 10.1523/JNEUROSCI.3107-18.2019
Funder
This work was supported by Grant-in-Aid for JSPS Research Fellow (to O.T.), CREST from JST, Grant-in-Aid for Scientific Research (B) from MEXT, Takeda Science Foundation and Cooperative Research of 'Network Joint Research Center for Materials and Devices' (to M.S.), and by Grant-in-Aid for Scientific Research on Innovative Areas from MEXT (To M.S. and T.S.).



 PAGE TOP
PAGE TOP