Abstract:
Both osteoblasts and osteoclasts are necessary for fracture repair. While osteoblasts are known to control the formation and activity of osteoclasts, the inter-cellular signaling involved in this process is not fully understood. Researchers led by Kanazawa University have now shown that osteoblast-derived extracellular vesicles containing signaling molecule RANKL are taken up by immature osteoclasts, triggering maturation. An understanding of osteoblast-osteoclast communication is the first step in developing effective therapies for metabolic and genetic bone diseases.
Kanazawa, Japan – In a study published recently in Communications Biology, researchers led by Kanazawa University explain how a tiny fish could help end painful metabolic and genetic bone diseases.
While pain, swelling, and immobility are the most obvious aftereffects of a broken bone, there is also a flurry of activity going on at the cellular level in an effort to repair the damage. And because bones are usually hidden away under layers of muscle, fat, and skin, it has been difficult for researchers to study in real time how bones regenerate.
“To provide insight into the process of fracture repair, we needed bone that was easily accessible and a model species in which bone growth mimicked that of humans,” says lead author Jingjing Kobayashi-Sun. “With transgenic and mutant lines already available, zebrafish scales ticked all the boxes.”
Zebrafish scales, although much simpler than mammalian bones, respond and develop in almost the same way as their more complex counterparts, and are conveniently located on the outside of the body. Most importantly though, they contain both osteoclasts and osteoblasts, the cells that respectively clear away broken bone fragments and generate new bone after a fracture.
Explains Kobayashi-Sun, “Recent studies have suggested that molecular packages, called extracellular vesicles (EVs), derived from osteoblasts may deliver signaling molecules to immature osteoclasts, triggering their differentiation into mature, active cells. However, given the difficulty of live imaging in bone, no one has been able to prove this process actually occurs in vivo.”
To visualize osteoblasts and osteoclasts in zebrafish scales, the researchers generated a double transgenic line in which the two cell types expressed different color fluorescent labels. After making small cuts in the scales of anesthetized fish, the researchers could identify the different cell types in the healing bone using a fluorescent microscope or flow cytometry.
“We observed that a large number of the green fluorescent osteoclasts at the fracture site contained red fluorescent osteoblast-derived particles in the cytoplasm, confirming the uptake of EVs by immature osteoclasts,” says senior author Isao Kobayashi. “In addition, mature osteoclasts were abundant in the damaged scales, indicating that EV uptake triggers the differentiation of osteoclasts.”
The EVs contained high levels of a cellular differentiation-associated signaling molecule called RANKL. Knocking out the corresponding gene resulted in a significant reduction in the number of osteoclasts, suggesting that osteoblasts control osteoclast formation via Rankl signaling.
Given that many bone disorders result from a breakdown in communication between osteoblasts and osteoclasts, these findings provide valuable information for the development of novel drug therapies.
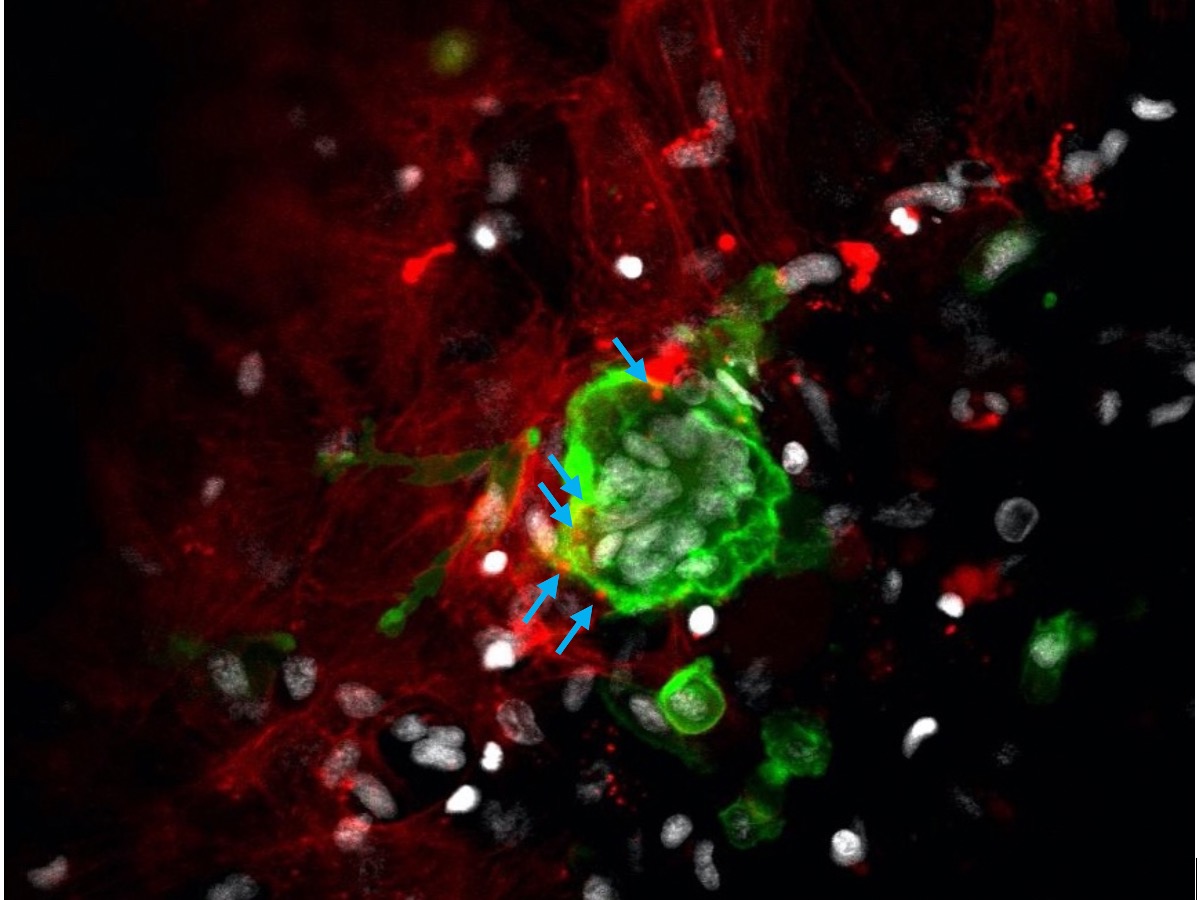
Figure. Zebrafish bone cells
Osteoclasts and osteoblasts in zebrafish scales were fluorescently labeled with GFP (green) and mCherry (red), respectively. A nucleus is shown in gray. Osteoclasts receive messages from osteoblasts via extracellular vesicles (blue arrows) and fuse to form a multinucleated giant cell in the bone tissue.
Article
Uptake of osteoblast-derived extracellular vesicles promotes the differentiation of osteoclasts in the zebrafish scale
Journal:Communications Biology
Authors:Jingjing Kobayashi-Sun, Shiori Yamamori, Mao Kondo, Junpei Kuroda, Mika Ikegame, Nobuo Suzuki, Kei-ichiro Kitamura, Atsuhiko Hattori, Masaaki Yamaguchi & Isao Kobayashi
DOI: 10.1038/s42003-020-0925-1
Funder
This work was supported in part by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (18K06331).



 PAGE TOP
PAGE TOP